��Ŀ����
12�� C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
C��N��O��Al��Si��Cu�dz���������Ԫ�أ���1��Siλ��Ԫ�����ڱ��������ڵ�IVA�壻
��2��N�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3��Cu�Ļ�̬ԭ���������1�����ӣ�
��3���á�����������գ�
| ԭ�Ӱ뾶 | �縺�� | �۵� | �е� |
| Al��Si | N��O | ���ʯ������� | CH4��SiH4 |
0��t1ʱ��ԭ��صĸ�����AlƬ����ʱ�������ĵ缫��Ӧʽ��2H++NO3-+e-=NO2��+H2O����Һ�е�H+�������ƶ���t1ʱ��ԭ����е��������������ı䣬��ԭ����Al��Ũ�����з����ۻ�������Ĥ��ֹ��Al�Ľ�һ����Ӧ��
���� ��1��Siԭ������Ϊ14����3�����Ӳ㣬����������Ϊ4��
��2��N��ԭ������Ϊ7�����Ų�3���ܲ㣻CuΪ29��Ԫ�أ��ͺ�������Ų�ʽΪ��[Ar]3d104s1���ݴ˽�ɣ�
��3��ͬһ���ڣ�ԭ������ԽС���뾶Խ��Ԫ�صķǽ�����Խǿ����縺��Խ����������ͬ�ģ�ԭ�Ӱ뾶ԽС���۵�Խ�ߣ�������ɺͽṹ���Ƶķ��Ӿ��壬��Է�������Խ�е�Խ�ߣ��ݴ˽�ɣ�
��4���������⣬0��t1ʱ��ԭ��صĸ�����AlƬ�����к���ɫ���������˵����Һ�е�������������ŵ磬�ݴ˽�ɣ����������ı䣬��ԭ��ص������������ı䣬�ݴ˷���ԭ�ɣ�
��� �⣺��1��Siԭ������Ϊ14����3�����Ӳ㣬����������Ϊ4����Si���ڵ������ڵ�IVA�壬�ʴ�Ϊ������IVA��
��2��N��ԭ������Ϊ7�����Ų�3���ܲ㣬���������Ų�ʽΪ��1s22s22p3��CuԪ��Ϊ29��Ԫ�أ�ԭ�Ӻ�����29�����ӣ����Ժ�������Ų�ʽΪ��1s22s22p63s23p63d104s1����������������Ϊ1���ʴ�Ϊ��1s22s22p3��1��
��3��ͬһ���ڣ�ԭ������ԽС���뾶Խ������ԭ������Al��Si���ʰ뾶Al��Si��Ԫ�صķǽ�����Խǿ����縺��Խ�����ڷǽ�����O��N���ʵ縺��N��O������������ͬ�ģ�ԭ�Ӱ뾶ԽС���۵�Խ�ߣ�����C��ԭ�Ӱ뾶С��Si��ԭ�Ӱ뾶�����۵㣺���ʯ������裻������ɺͽṹ���Ƶķ��Ӿ��壬��Է�������Խ�е�Խ�ߣ�����SiH4��Է�����������CH4���ʷе�CH4��SiH4���ʴ�Ϊ������������������
��4��0��t1ʱ��ԭ��صĸ�����AlƬ����Һ�в�������ɫ�����Ƕ�����������������Ӧ����ʽΪ��2H++NO3-+e-=NO2��+H2O����ʱ��Һ�е�����������������һ��ʱ�������Al��Ũ���ᷢ���ۻ�������ԭ�����Al��������Cu���������ʴ�Ϊ��2H++e-+NO3-=NO2��+H2O������Al��Ũ�����з����ۻ�������Ĥ��ֹ��Al�Ľ�һ����Ӧ��
���� ������Ҫ�����Ԫ��λ���ԵĹ�ϵ��ԭ��ع���ԭ�����漰�縺�Դ�С�Ƚϡ�ԭ�Ӱ뾶��С�Ƚϵȣ��ѶȲ���ע��������

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�| A�� | A=1��10-4B | B�� | B=1��10-4A | C�� | A��B | D�� | A=B |
| A�� | �ڼ�����Һ�У�Na+��S2-��K+��AlO2- | |
| B�� | ��������Һ�У�SO32-��S2-��K+��NH4+ | |
| C�� | ��������Һ�У�Fe2+��Cu2+��Cl-��NO3- | |
| D�� | ��������Һ�У�Fe2+��Cl-��Na+��SO42- |
| A�� | 23gNa������H2O��Ӧ��ȫ�������NA��H2���� | |
| B�� | 1molCu��������Ũ���ᷴӦ������NA��SO3���� | |
| C�� | ��״���£�22.4LN2��H2������к�NA��ԭ�� | |
| D�� | 3mol����Fe��ȫת��ΪFe3O4��ʧȥ8NA������ |
��1����NH4Cl��Ca��OH��2�Ʊ�NH3����Ӧ�����������ռ���β������װ������ΪACG��

��2����ͼװ�ý���NH3����ʵ�飮

���ȴ�����1��Bƿ�е������dz��ְ��̣�ԭ����Aƿѹǿ��Bƿ���Ȼ������Bƿ�백����Ӧ�����Ȼ�粒���С�����γɰ��̣��ȶ��ر�����1��
���ٴ�����2��Bƿ�е���������ƿ�е�Һ�嵹����Bƿ������ɫʯ����Һ��죮
��3�����ʵ�飬̽��ijһ�����ض���Һ��NH4Clˮ��̶ȵ�Ӱ�죮
��ѡ�Լ�������������NH4Cl������ˮ��100mL����ƿ���ձ�����ͷ�ιܡ���������ҩ�ס���ƽ��pH�ơ��¶ȼơ�����ˮԡ�ۣ��ɵ����¶ȣ�
��ʵ��Ŀ�ģ�̽���¶ȶ���Һ��NH4Clˮ��̶ȵ�Ӱ�죮
�����ʵ�鷽�����ⶨʵ�������������ʵ�鷽�����г���ֱ�Ӷ�ȡ���ݵ���������������ⶨ�����ݣ���������ĸ��ʾ�����С�V����Һ������ʾ��������Һ���������
| ������ ʵ����� | V����Һ��/ml | �� | |||
| 1 | 100 | �� | |||
| 2 | 100 | �� |
| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| B�� | 1L 0.1mol•L-1��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | 235g����92235U�����ѱ䷴Ӧ��92235U+01n$\stackrel{�ѱ�}{��}$3890Sr+54136Xe+1001n�����������ӣ�01n����Ϊ10NA |
| A�� | ���ʵķе㣺W��X | B�� | �����ӵĻ�ԭ�ԣ�W��Z | ||
| C�� | �������ˮ��������ԣ�Y��Z | D�� | X��Y���ܴ�����ͬһ���ӻ������� |
| A�� | 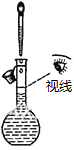 ����һ��Ũ�ȵ�NaCl��Һ | B�� | 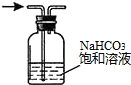 ��ȥ�����е�HCl���� | ||
| C�� | 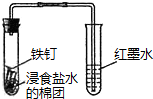 �۲�����������ʴ | D�� | 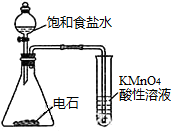 ������Ȳ�Ļ�ԭ�� ������Ȳ�Ļ�ԭ�� |

 ����ƽ���ͷ���
����ƽ���ͷ���