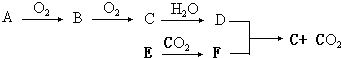��Ŀ����
17��NA���������ӵ�������ֵ������������ȷ���ǣ�������| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| B�� | 1L 0.1mol•L-1��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | 235g����92235U�����ѱ䷴Ӧ��92235U+01n$\stackrel{�ѱ�}{��}$3890Sr+54136Xe+1001n�����������ӣ�01n����Ϊ10NA |
���� A�������к�7��C-H����2��C-C����1��C-O����1��O-H����
B��HCO3-ˮ������̼�ᣬ���ԭ���غ������
C���Ʒ���������Ӧ��NaԪ�صĻ��ϼ���0����Ϊ+1�ۣ�
D��92235U+01n$\stackrel{�ѱ�}{��}$3890Sr+54136Xe+1001n������������Ϊ10-1=9����
��� �⣺A.60g����Ϊ1mol�������к�7��C-H����2��C-C����1��C-O����1��O-H�������ڵĹ��ۼ�����Ϊ11NA����A����
B.1L 0.1mol•L-1��NaHCO3��Һ��HCO3-��CO32-������֮��С��0.1NA��̼������к�Cԭ�ӣ���B����
C.23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ$\frac{23g}{23g/mol}$����1-0����NA=1NA����C��ȷ��
D��92235U+01n$\stackrel{�ѱ�}{��}$3890Sr+54136Xe+1001n������������Ϊ10-1=9������235g����92235U�����ѱ䷴Ӧ�����������ӣ�01n����Ϊ9NA����D����
��ѡC��
���� ���⿼�鰢��٤�����������㣬Ϊ��Ƶ���㣬���������еĻ�ѧ���������غ㡢����ˮ�⡢������ԭ��Ӧ��ת�Ƶ��Ӽ����Ϊ���Ĺؼ������ط�����Ӧ���������ۺϿ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
�����Ŀ
5����O2��HClת��ΪCl2�������Ч�棬������Ⱦ��

��1����ͳ�ϸ�ת��ͨ����ͼ1��ʾ�Ĵ�ѭ��ʵ�֣����У���Ӧ��Ϊ��2HCl��g��+CuO��s��?H2O��g��+CuCl2��s����H1����Ӧ������1molCl2��g���ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ4HCl��g��+O2��g��=2Cl2��g��+2H2O��g����H=2����H1+��H2������Ӧ���á�H1�͡�H2��ʾ����
��2������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԣ�
��ʵ������һ��ѹǿ�£��ܷ�Ӧ��HClƽ��ת�������¶ȱ仯�Ħ�HCl��T������ͼ2�����ܷ�Ӧ�ġ�H��0�����������=����������A��B�����ƽ�ⳣ��K��A����K��B���нϴ����K��A����
��������ʵ������ѹ�����ʹѹǿ��������Ӧ��HCl��T���ߵ�ʾ��ͼ������Ҫ˵�����ɣ�����ѹǿ��ƽ��������Ӧ�����ƶ�����HCl������ͬ�¶���HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�
�����д�ʩ�У���������ߦ�HCl����BD��
A������n��HCl�� B������n��O2��
C��ʹ�ø��õĴ��� D����ȥH2O
��3��һ�������²�÷�Ӧ������n��Cl2�����������£�
����2.0��6.0min����HCl�����ʵ����仯��ʾ�ķ�Ӧ���ʣ���mol•min-1Ϊ��λ��д��������̣���
��4��Cl2��;�㷺��д����Cl2�Ʊ�Ư�۵Ļ�ѧ��Ӧ����ʽ2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��

��1����ͳ�ϸ�ת��ͨ����ͼ1��ʾ�Ĵ�ѭ��ʵ�֣����У���Ӧ��Ϊ��2HCl��g��+CuO��s��?H2O��g��+CuCl2��s����H1����Ӧ������1molCl2��g���ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ4HCl��g��+O2��g��=2Cl2��g��+2H2O��g����H=2����H1+��H2������Ӧ���á�H1�͡�H2��ʾ����
��2������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԣ�
��ʵ������һ��ѹǿ�£��ܷ�Ӧ��HClƽ��ת�������¶ȱ仯�Ħ�HCl��T������ͼ2�����ܷ�Ӧ�ġ�H��0�����������=����������A��B�����ƽ�ⳣ��K��A����K��B���нϴ����K��A����
��������ʵ������ѹ�����ʹѹǿ��������Ӧ��HCl��T���ߵ�ʾ��ͼ������Ҫ˵�����ɣ�����ѹǿ��ƽ��������Ӧ�����ƶ�����HCl������ͬ�¶���HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�
�����д�ʩ�У���������ߦ�HCl����BD��
A������n��HCl�� B������n��O2��
C��ʹ�ø��õĴ��� D����ȥH2O
��3��һ�������²�÷�Ӧ������n��Cl2�����������£�
| t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
| n��Cl2��/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
��4��Cl2��;�㷺��д����Cl2�Ʊ�Ư�۵Ļ�ѧ��Ӧ����ʽ2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
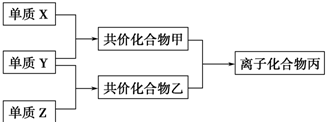
12�� C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
��1��Siλ��Ԫ�����ڱ��������ڵ�IVA�壻
��2��N�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3��Cu�Ļ�̬ԭ���������1�����ӣ�
��3���á�����������գ�
��4�������£�����ȥ��������Ĥ��Al��CuƬ����ŨHNO3�����ԭ��أ�ͼ1�������ԭ��صĵ���ǿ�ȣ�I����ʱ�䣨t���ı仯��ͼ2��ʾ����Ӧ�������к���ɫ���������
0��t1ʱ��ԭ��صĸ�����AlƬ����ʱ�������ĵ缫��Ӧʽ��2H++NO3-+e-=NO2��+H2O����Һ�е�H+�������ƶ���t1ʱ��ԭ����е��������������ı䣬��ԭ����Al��Ũ�����з����ۻ�������Ĥ��ֹ��Al�Ľ�һ����Ӧ��
 C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
C��N��O��Al��Si��Cu�dz���������Ԫ�أ���1��Siλ��Ԫ�����ڱ��������ڵ�IVA�壻
��2��N�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3��Cu�Ļ�̬ԭ���������1�����ӣ�
��3���á�����������գ�
| ԭ�Ӱ뾶 | �縺�� | �۵� | �е� |
| Al��Si | N��O | ���ʯ������� | CH4��SiH4 |
0��t1ʱ��ԭ��صĸ�����AlƬ����ʱ�������ĵ缫��Ӧʽ��2H++NO3-+e-=NO2��+H2O����Һ�е�H+�������ƶ���t1ʱ��ԭ����е��������������ı䣬��ԭ����Al��Ũ�����з����ۻ�������Ĥ��ֹ��Al�Ľ�һ����Ӧ��
9�������й����ʵıȽϣ�������Ԫ�������ɽ��͵��ǣ�������
| A�� | ���ԣ�H2SO4��H3PO4 | B�� | �ǽ����ԣ�Cl��Br | ||
| C�� | ���ԣ�NaOH��Mg��OH��2 | D�� | ���ȶ��ԣ�Na2CO3��NaHCO3 |
6���л��������Ź�Բ��õķ���ʷ�����з������첻�漰��ѧ��Ӧ���ǣ�������
| A�� | �õ�����ͭ | B�� | ������ʯ���� | ||
| C�� | �ս�ճ�����մ� | D�� | ��ĥ��ʯ��ָ���� |
 ��
��
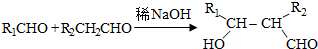
 ��
�� ��
��
 ��д�ṹ��ʽ��
��д�ṹ��ʽ��