��Ŀ����
17��ijʵ��С���ù�ҵ�Ϸ�������Ҫ�ɷ�Cu2S��Fe2O3����ȡ��ͭ���̷���FeSO4•7H2O����Ʒ������������£�
��1����ʵ�����У�����98%��Ũ���ᣨ�ܶ�Ϊ1.84g•mL-1������500mL1.0mol•L-1�����ᣬ��Ҫ����������Ͳ���ձ����������⣬���н�ͷ�ιܡ�500mL����ƿ��
��2����С��ͬѧ�������װ��ģ������ڹ��������б��գ�����֤�����к���Ԫ�أ�
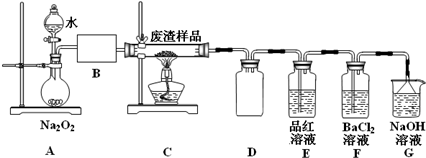
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����Ϊ���Ʒ�Ӧ�����ڼ��Ҳ�����ƽ����������ȡ�IJ����������Ǵ�Һ©���Ͽڻ��������Ʒ�Һ©��������ʹˮ������ε��£�B��Ӧ����ʢ�м�ʯ�ҵĸ���ܣ���U�ιܣ���Ũ�����ϴ��ƿ����д�Լ����������ƣ���
��Eװ���м���Ʒ����Һ��Ŀ���Ǽ�������a�е�SO2����Fװ���г��ְ�ɫ����ʱ����Ӧ���ӷ���ʽΪ2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+��
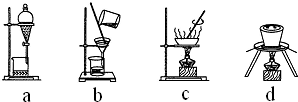
��3�����в����У������ڲ�����н��еIJ�������ad�������и�������ţ���
��������ɴ�ͭ�õ���ͭ�ķ���Ϊ��⾫������д���ƣ���
��4��Ϊ�ⶨ��Ʒ���̷���������������ȡ30.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ�����ӦΪ��10FeSO4+8H2SO4+2KMnO4=2MnSO4+5Fe2��SO4��3+K2SO4+8H2O��ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.90 | 20.02 | 19.98 | 20.00 |
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ���
d���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ò�Ʒ���̷�����������Ϊ92.7%��
���� ����������Ҫ�ɷ�Cu2S��Fe2O3�����շ������巢����ӦCu2S+2O2$\frac{\underline{\;����\;}}{\;}$SO2+2CuO����������a��SO2������A��CuO��Fe2O3������A��ϡ�����ϣ������ķ�ӦΪCuO+H2SO4=CuSO4+H2O��Fe2O3+3H2SO4=Fe2��SO4��3+3H2O��������ҺA�гɷ�ΪCuSO4��Fe2��SO4��3������ҺA�м������Feм��������ӦCuSO4+Fe=FeSO4+Cu��2Fe2��SO4��3+Fe=3FeSO4�����Թ���B�ɷ���Fe��Cu����ҺB�ɷ�ΪFeSO4������B��ϡ�����ϣ�Fe��ϡ���ᷴӦ����FeSO4��Cu��ϡ�����Ӧ�����˵õ������ͭ���ݴ˷������
��� �⣺��1������98%��Ũ���ᣨ�ܶ�Ϊ1.84g•mL-1������500mL1.0mol•L-1�����ᣬ��Ҫ����������Ͳ���ձ������������ζ��ﵽ�̶���ʱ�ý�ͷ�ιܣ�������500mL����ƿ�н��У��ʴ�Ϊ����ͷ�ιܡ�500mL����ƿ��
��2����װ��A�з�Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����ͨ������ˮ�����������Ʒ�Ӧ�����ʣ����Կ��Դ�Һ©���Ͽڻ��������Ʒ�Һ©��������ʹˮ������ε��£�Bװ��Ӧ�Ǹ���װ�ã������ü�ʯ�ҵĸ���ܣ���U�ιܣ���Ũ�����ϴ��ƿ���ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2������Һ©���Ͽڻ��������Ʒ�Һ©��������ʹˮ������ε��£���ʯ�ҵĸ���ܣ���U�ιܣ���Ũ�����ϴ��ƿ��
��Eװ���м���Ʒ����Һ��Ŀ���Ǽ�������a�е�SO2����δ��Ӧ�����������������ˮ��Һ�з���������ԭ��Ӧ��������������ӣ��뱵���ӽ���������ᱵ��ɫ���������Է�Ӧ����ʽΪ��2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+���ʴ�Ϊ����������a�е�SO2��2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+��
��3����������ڻ�ԭ����ϵ������Ũ���ᾧ���ˣ������漰��װ��Ϊ��bc�������ڵ���ad����ͭ�õ���ͭ���õ�⾫�����ʴ�Ϊ��ad�� ��⾫������������
��4���ٵ�1��ʵ�����ݳ����쳣�����ĸ��������Һ������
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ����Һ��Ũ�ȱ�С�����ĸ��������Һ��������ȷ��
b����ƿϴ����δ�����Ӱ�죬�ʴ���
c���ζ��յ�ʱ���Ӷ��������������Һ�����С���ʴ���
d���ζ��յ�ʱ���Ӷ��������������Һ��������ȷ��
��ѡ��ad��
�ڵ�2��3��4����ƽ��ֵ������KMnO4��Һ���Ϊ��$\frac{20.02+19.98+20.00}{3}$=20ml����10FeSO4+8H2SO4+2KMnO4=2MnSO4+5Fe2��SO4��3+K2SO4+8H2O�ã�
10FeSO4����2KMnO4��
10��152 2
m 20ml��0.1000mol•L-1��10-3
m=$\frac{10��152��20ml��0.1000mol•{L}^{-1}��1{0}^{-3}}{2}$=1.52g�������̷������ʵ�Ϊ��Ϊ��$\frac{1.52g}{152g/mol}��\frac{250}{25}$=0.1mol�������̷�����������Ϊ��$\frac{0.1����152+126��}{30}$��100%=92.7%���ʴ�Ϊ��92.7%��
���� ���⿼������ķ�����ᴿ�����ؿ���ѧ���������⡢ʵ�������˼ά�����Ե���������ȷ���ʵ������ǽⱾ��ؼ����ܴ������Ϸ����������跢���ķ�Ӧ������ȷ��д��Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | V��B2��=0.8mol/��L•s�� | B�� | V��A2��=0.8mol/��L•s�� | ||
| C�� | V��C��=0.6mol/��L•s�� | D�� | V��A2��=1.8mol/��L•min�� |
| A�� | ������������ | |
| B�� | ��������Ȼ������Ҫ�ɷ� | |
| C�� | �������������ڹ��������·�����Ӧ | |
| D�� | ������ʹ������Ȼ�̼��Һ�����Ը��������Һ��ɫ |
| A�� | ��ȡ8.0gCuSO4������500mLˮ | |
| B�� | ��ȡ7.68gCuSO4������480mLˮ | |
| C�� | ��ȡ12.5gCuSO4•5H2O����ˮ���500mL��Һ | |
| D�� | ��ȡ12.0gCuSO4•5H2O����ˮ���480mL��Һ |
| A�� | Na+��Mg2+��Al3+�����������μ��� | B�� | RbOH��KOH��Mg��OH��2�ļ������μ��� | ||
| C�� | H2S��H2O��HF���ȶ���������ǿ | D�� | H4SiO4��H2SO4��HClO4����������ǿ |
| A�� | ������ᡢ��������������ظ������� | |
| B�� | ij����ˮ����һ��ʱ�䣬��pH��4.68��Ϊ4.28����Ϊˮ���ܽ��˽϶��CO2 | |
| C�� | �ƾ������õ��л��ܼ�����ϴȥƤ������մ�еı��� | |
| D�� | Ư����ˮ��Һ���ܹ����ɴ����ᣬ������Ư��ֽ�� |
| A�� | �ڻ�ѧ��Ӧ�У��Ͽ���ѧ��Ҫ�����������γɻ�ѧ��Ҫ�ų����� | |
| B�� | ��ѧ��Ӧ���������������⣬�������������ı仯 | |
| C�� | ����Ӧ������������������������������÷�Ӧ��Ϊ���ȷ�Ӧ | |
| D�� | ���ȷ�Ӧ�Ƿ��ȷ�Ӧ |
 ��
��