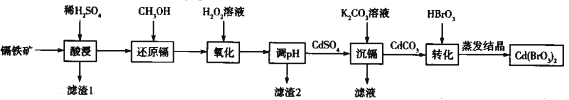
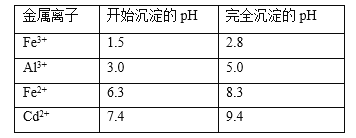
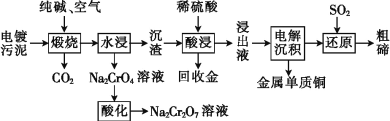
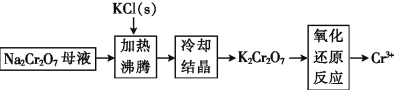
��Ŀ����
����Ŀ���ڸ����£� Al��Fe2O3�������ȷ�Ӧ��õ��Ĺ�����������Ҫ����Al2O3��Fe������������Fe2O3���Ӹ���Ʒ�й�����������Al2O3��������Fe��Fe2O3���������£�

��֪��NaAlO2 + CO2 + 2H2O = Al��OH��3�� + NaHCO3
�ش��������⣺
��1������ٵijɷ���__________����Һ�ڵ�������____________��
��2���������NaOH��Һʱ��������Ӧ�����ӷ���ʽ��__________��
��3����ɫ�������NaOH��Һ��Ӧ�����ӷ���ʽ��__________��
���𰸡�Fe��Fe2O3 NaHCO3 Al2O3 + 2OH-= 2AlO2- + H2O Al��OH��3 + OH-= AlO2- + 2H2O
��������
��������ӹ���NaOH��Һ�õ���Һ��ΪNaAlO2��NaOH�Ļ����Һ�������ΪFe��Fe2O3����Һ��ͨ������CO2�õ���Һ��ΪNaHCO3��Һ�������ΪAl��OH��3�������Դ˴��⡣
��������ӹ���NaOH��Һ�õ���Һ��ΪNaAlO2��NaOH�Ļ����Һ�������ΪFe��Fe2O3����Һ��ͨ������CO2�õ���Һ��ΪNaHCO3��Һ�������ΪAl��OH��3������Al��OH��3���ȷֽ�õ�Al2O3��
��1������ٵijɷ���Fe��Fe2O3����Һ�ڵ�����NaHCO3���ʴ�Ϊ��Fe��Fe2O3��NaHCO3��
��2���������NaOH��Һʱ��������Ӧ�����ӷ���ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3����ɫ�����ΪAl��OH��3��NaOH��Һ��Ӧ�����ӷ���ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

����Ŀ���ס��ҡ�����ȡ300mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһþ���Ͻ��ĩ��������ʵ�飬�й������б����£�
ʵ����� | �� | �� | �� |
�Ͻ�����/mg | 510 | 765 | 918 |
(��״��)�������/mL | 560 | 672 | 672 |
��1����������ʵ���Ũ���Ƕ���___��
��2���Ͻ���þ���������������Ƕ���___��___��