题目内容
【题目】钢是现代社会的物质基础,钢中除含有铁外还含有碳和少量不可避免的钴、硅、锰、磷、硫等元素。请回答下列有关问题:
(1)基态Mn原子的价电子排布式为___________。Mn2+与Fe2+中,第一电离能较大的是__________,判断的理由是_____________________________________。
(2)碳元素除可形成常见的氧化物CO、CO2外,还可形成C2O3(结构式为![]() )。C2O3中碳原子的杂化轨道类型为___________,CO2分子的立体构型为___________。
)。C2O3中碳原子的杂化轨道类型为___________,CO2分子的立体构型为___________。
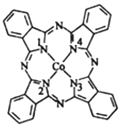
(3)酞菁钴分子的结构简式如图所示,分子中与钴原子通过配位键结合的氮原子的编号是______(填“1” “2” “3”或“4”)其中C、H、O元素电负性由大到小的顺序是_________________________
(4)碳酸盐的热分解是由于晶体中的阳离子结合碳酸根中的氧离子,是碳酸根分解为CO2分子的结果。MgCO3分解温度低于CaCO3,请解释原因_________________________。
(5)氧化亚铁晶胞与NaCl的相似,NaCl的晶胞如图所示。由于晶体缺陷,某氧化亚铁晶体的实际组成为Fe0.9O,其中包含有Fe2+和Fe3+,晶胞边长为apm,该晶体的密度为ρg·cm-3,则a=___________(列出计算式即可,用NA表示阿伏加德罗常数的值)。

【答案】3d54s2 Mn2+ Mn2+价层电子排布为3d5,3d能级半充满,更稳定 sp2 直线型 2,4 O>C>H 半径Mg2+<Ca2+,MgO晶格能大于CaO, Mg2+更易结合碳酸根中的氧离子,故MgCO3更易分解 ![]()
【解析】
(1)Mn位于第四周期ⅦB,其价电子包括最外层电子和次外层的d能级,即Mn的价电子排布式为3d54s2;Mn2+价电子排布式为3d5,Fe2+价电子排布式为3d6,Mn2+价层电子排布中3d能级半充满,更稳定,因此第一电离能较大的是Mn2+;
(2)根据C2O3的结构式,C有3个σ键,无孤电子对,因此C的杂化类型为sp2;CO2中C有2个σ键,孤电子对数为(4-2×2)/2=0,即CO2杂化类型为sp,CO2空间构型为直线型;
(3)N形成3个共价键达到饱和,若N有4个化学键,其中有一个是配位键,根据结构简式,2,4含有配位键,非金属性O>C>H,则C、H、O的电负性大小顺序是O>C>H;
(4)MgCO3和CaCO3都属于离子晶体,受热分解后生成MgO和CaO,也都属于离子晶体,MgCO3分解温度低于CaCO3,应从晶格能的角度分析,原因是半径Mg2+<Ca2+,MgO晶格能大于CaO, Mg2+更易结合碳酸根中的氧离子,故MgCO3更易分解;
(5)根据氯化钠晶胞,NaCl的晶胞中有4个“NaCl”,Fe0.9O晶胞与NaCl晶胞的相似,因此该晶体中有4个“Fe0.9O”,晶胞的质量为![]() ,晶胞的体积(a×10-10)cm3,根据密度的定义,ρ=
,晶胞的体积(a×10-10)cm3,根据密度的定义,ρ=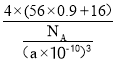 ,得出a=
,得出a=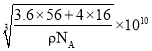 。
。

 名校名师培优作业本加核心试卷系列答案
名校名师培优作业本加核心试卷系列答案 全程金卷系列答案
全程金卷系列答案【题目】一定温度下,在三个容积均为2.0 L的恒容密闭容器中发生反应:2NO(g)+2CO(g)![]() N2(g)+2CO2(g)。各容器中起始物质的量与反应温度如下表所示,反应过程中甲、丙容器中CO2的物质的量随时间变化关系如图所示:
N2(g)+2CO2(g)。各容器中起始物质的量与反应温度如下表所示,反应过程中甲、丙容器中CO2的物质的量随时间变化关系如图所示:

容器 | 温度/℃ | 起始物质的量/mol | |
NO (g) | CO (g) | ||
甲 | T1 | 0.20 | 0.20 |
乙 | T1 | 0.30 | 0.30 |
丙 | T2 | 0.20 | 0.20 |
下列说法正确的是
A. 该反应的正反应为吸热反应
B. 达到平衡时,乙中CO2的体积分数比甲中的小
C. T1℃时,若起始时向甲中充入0.40 mol NO、0.40mol CO、0.40mol N2和0.40mol CO2,则反应达到新平衡前v(正)<v(逆)
D. T2℃时,若起始时向丙中充入0.06molN2和0.12 molCO2,则达平衡时N2的转化率大于40%