��Ŀ����
4��ij��A������������ܶ�Ϊ35��һ����A����������������ȫȼ�գ��õ������ʵ�����CO2��H2O��A�ܷ�������ת����ͼ�е������������ȥ��������F�ܷ���������Ӧ��E���ܷ���������Ӧ��G��H��Ϊͬ���칹�壬G��ֻ��1��������֪��
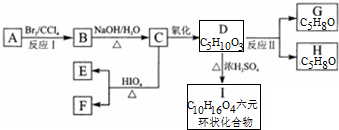
��1����ϵͳ��������A����2-��-1-��ϩ��
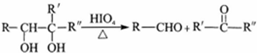
��2��C��HIO4�ķ�Ӧ������������Ӧ��
��3��д��F��G�Ľṹ��ʽ��FHCHO��GCH2=C��COOH��CH2CH3��
��4��д��B��C��D��I�Ļ�ѧ����ʽ��
B��CCH2BrCBr��CH3��CH2CH3+2NaOH$��_{��}^{H_{2}O}$CH2OHCOH��CH3��CH2CH3+2NaBr��
D��I2HOOCCOH��CH3��CH2CH3$��_{��}^{Ũ����}$
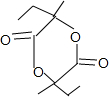 +2H2O��
+2H2O����5��I�ж���ͬ���칹�壬д�������������������е�ͬ���칹�壺����Ԫ�����ڻ��ϵ�һ����ȡ����ֻ��һ�֣���1 mol��������������NaHCO3���÷ų�2 mol���壻�ܺ˴Ź�������Ϊ3��壮���������Ľṹ��ʽ�������������칹��
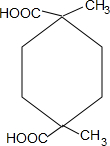 ��
��
���� ij��A������������ܶ�Ϊ35����������Է�������Ϊ70��һ����A����������������ȫȼ�գ��õ������ʵ�����CO2��H2O��˵��CHԭ�Ӹ�����Ϊ1��2����A�ķ���ʽΪC5H10������֪��Ϣ��F�ܷ���������Ӧ��E���ܷ���������Ӧ��֪��C����������D��D�ķ���ʽΪC5H10O3�����ܷ���������������Ӧ������I������AΪCH2=C��CH3��CH2CH3����BΪCH2BrCBr��CH3��CH2CH3��CΪCH2OHCOH��CH3��CH2CH3��EΪCH3COCH2CH3��FΪHCHO��C��������D��DΪHOOCCOH��CH3��CH2CH3����GH�ķ���ʽ��֪��D��GH�ķ�Ӧ�Ǵ��ǻ�����ȥ��Ӧ��G��ֻ��1������GΪCH2=C��COOH��CH2CH3��D����������������Ӧ������I��IΪ ���ݴ˷�����
���ݴ˷�����
��� �⣺ij��A������������ܶ�Ϊ35����������Է�������Ϊ70��һ����A����������������ȫȼ�գ��õ������ʵ�����CO2��H2O��˵��CHԭ�Ӹ�����Ϊ1��2����A�ķ���ʽΪC5H10������֪��Ϣ��F�ܷ���������Ӧ��E���ܷ���������Ӧ��֪��C����������D��D�ķ���ʽΪC5H10O3�����ܷ���������������Ӧ������I������AΪCH2=C��CH3��CH2CH3����BΪCH2BrCBr��CH3��CH2CH3��CΪCH2OHCOH��CH3��CH2CH3��EΪCH3COCH2CH3��FΪHCHO��C��������D��DΪHOOCCOH��CH3��CH2CH3����GH�ķ���ʽ��֪��D��GH�ķ�Ӧ�Ǵ��ǻ�����ȥ��Ӧ��G��ֻ��1������GΪCH2=C��COOH��CH2CH3��D����������������Ӧ������I��IΪ ��
��
��1��AΪCH2=C��CH3��CH2CH3��������Ϊ2-��-1-��ϩ���ʴ�Ϊ��2-��-1-��ϩ��
��2��CΪCH2OHCOH��CH3��CH2CH3������ϢC��HIO4�ķ�Ӧ������������Ӧ���ʴ�Ϊ��������Ӧ��
��3��FΪHCHO��GΪCH2=C��COOH��CH2CH3���ʴ�Ϊ��HCHO��CH2=C��COOH��CH2CH3��
��4��BΪCH2BrCBr��CH3��CH2CH3������NaOH��Һ��ˮ������C��CΪCH2OHCOH��CH3��CH2CH3����Ӧ����ʽΪCH2BrCBr��CH3��CH2CH3+2NaOH$��_{��}^{H_{2}O}$CH2OHCOH��CH3��CH2CH3+2NaBr��DΪHOOCCOH��CH3��CH2CH3��D����������������Ӧ������I��IΪ ����Ӧ�Ļ�ѧ����ʽΪ2HOOCCOH��CH3��CH2CH3$��_{��}^{Ũ����}$
����Ӧ�Ļ�ѧ����ʽΪ2HOOCCOH��CH3��CH2CH3$��_{��}^{Ũ����}$ +2H2O���ʴ�Ϊ��CH2BrCBr��CH3��CH2CH3+2NaOH$��_{��}^{H_{2}O}$CH2OHCOH��CH3��CH2CH3+2NaBr��2HOOCCOH��CH3��CH2CH3$��_{��}^{Ũ����}$
+2H2O���ʴ�Ϊ��CH2BrCBr��CH3��CH2CH3+2NaOH$��_{��}^{H_{2}O}$CH2OHCOH��CH3��CH2CH3+2NaBr��2HOOCCOH��CH3��CH2CH3$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
��5��IΪ ����ͬ���칹��1 mol��������������NaHCO3���÷ų�2 mol���壬˵�������к���2���Ȼ������������Ϣ��֪����ͬ���칹��Ϊ
����ͬ���칹��1 mol��������������NaHCO3���÷ų�2 mol���壬˵�������к���2���Ȼ������������Ϣ��֪����ͬ���칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע������л������ʽ��ȷ�����ƶ�ʱע�����ͻ�ƿڣ������л�������ŵ������Լ�ת�����ر��������Ϣ���ڽ��ʱҪע�����⣮

| A�� | ԭ��������W��M��X��Y��Z | B�� | ԭ�Ӱ뾶��Z��W��M��Y��X | ||
| C�� | ���Ӱ뾶��W2+��Z+��M-��Y2- | D�� | ����⻯������ȶ��ԣ�M��Y��X |

| A�� | ���������ķ���ʽC10H11O2 | |
| B�� | 1mol������ˮ����Ҫ����2mol NaOH | |
| C�� | ���������Է����ӳɡ�ȡ������ȥ��Ӧ | |
| D�� | ��������һ�ȴ��������� |
| A�� | H2OΪֱ���ͷ��� | |
| B�� | ��Ӧ��ÿ����1molSת����2mol���� | |
| C�� | NaHS�к����Ӽ��ͷǼ��Լ� | |
| D�� | ���ʣ�S8��Ϊԭ�Ӿ��� |
| ����� | �Լ� | ���뷽�� | |
| A | Fe���⣩ | - | ���� |
| B | ������̼���Ȼ��⣩ | ����Na2CO3��Һ | ϴ�� |
| C | �������������ᣩ | NaOH��Һ | ���� |
| D | ���ۣ��Ȼ��ƣ� | ����ˮ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��CoCl2��Һ���ݹ������Ƴɼ���ʪ�ȼƣ������ڼ��У��Դ��Թ��ƿ�����ʪ�� | |
| B�� | ��1mL0.5mol/L��AlCl3��Һ�м���2mL���͵�NaF��Һ���ټ���1mL3��mol/L�İ�ˮ���������̶����������� | |
| C�� | ��1mL1%��NaOH��Һ�м���2ml2%��CuSO4��Һ�����ټ���0.5mL�л���X����Һ�������ɫ����X�Ǹ��� | |
| D�� | �ñ�NaOH��Һ�ζ�����ʳ����Һʱ����ƿˮϴ��δ�ô���Һ��ϴ����ʽ�ζ��ܼ��첿�������ݣ��ζ���������ʧ����ⶨ���ƫ�� |
| A�� | 1.8g��ˮ�к��еĵ�����ΪNA | |
| B�� | 1L0.1mol/L FeCl3��Һ�к��е�Cl- ��Ϊ0.3NA | |
| C�� | 25�棬1L pH=13��Ba��OH��2��Һ�к��е�oH-��Ϊ0.2NA | |
| D�� | ��״���£�2.24L NO��NO2��������к��е���ԭ����Ϊ0.15NA |
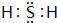 ��
��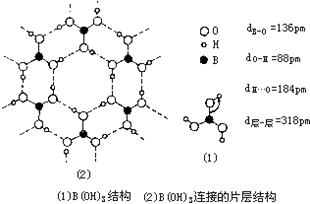 ���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ��
���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ��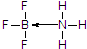 ��
��