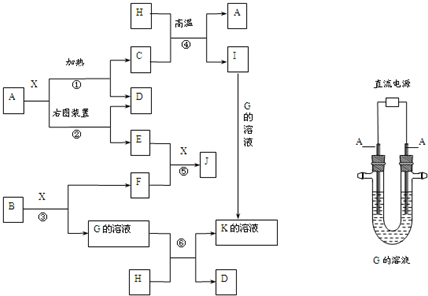
��Ŀ����
9��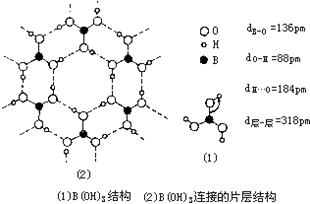 ���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ��
���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ����1����ԭ�ӵĵ����Ų�ʽ��1s22s22p1��
��2�����������B2H6�������飩���ṹ��ͼ������Bԭ�ӵ��ӻ���ʽΪsp3�ӻ���
��3��BF3��BCl3����ǿ�ҽ��ܹµ��ӶԵ��������������������백���������������ɰ�ɫ���壬д���ð�ɫ����ṹʽ������ע�����е���λ��
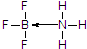 ��
����4�������������ǿ϶������˺Ͷ�����ж�����Ҫ�ⶾ�����������ڿ�����γ��ȶ��������BF4-
�������Ժͷ���ͬ��;���μ����ڴ�л�������Աȷ�С�����������ų�������Ϊ��Է�������нⶾ���ã�
��5�����ṹ�о�֤�������ᾧ����B��OH��3��Ԫ�ṹ��ͼ��1����ʾ������Ԫ�е���ԭ��ͨ��O-H��O�������ɲ�״�ṹ��ͼ��2����ʾ�������֮�������ķ��Ӽ������Ϲ����������ᾧ�壮
��H3BO3��һԪ���ᣬд������ˮ��Ӧ�Ļ�ѧ����ʽH3BO3+H2O?[B��OH��4]-+H+��
�ڸ��ݽṹ�ж�����˵����ȷ����bcdg��
a�����ᾧ������ԭ�Ӿ��� b�����ᾧ������Ƭ״�����
c�����ᾧ���Ƿ��Ӿ��� d�����ᾧ���л���У�������
e����B��OH��3��Ԫ�У�Bԭ����sp3�ӻ��������ԭ�ӽ�϶���
f��H3BO3���ӵ��ȶ���������й�
g����1mol H3BO3�ľ�������3mol���
h����������ԭ�������Ϊ8e-�ȶ��ṹ
�����᳣����Ϊ��ɫƬ״���壬����ˮ��273Kʱ�ܽ��Ϊ6.35��������ˮ���ܽ����������373KʱΪ27.6�������������ԭ������ʱ���ᾧ���еĴ�������в��ֶ������£���������������������ܽ⣮
���� ��1������Ԫ�����ƣ��ж�Ԫ��ԭ�ӵĺ�����������ٸ��ݺ�������Ų�������д����Ԫ��Ϊ5��Ԫ�أ�
��2�����ݼ۲���ӶԻ�������ȷ�����ӻ����ͣ�
��3����Ԫ�ؾ���ȱ�����ԣ��仯���������йµ��ӶԵķ��ӻ������γ�����BF3����NH3��Ӧ����BF3•NH3��Nԭ�Ӻ��йµ��Ӷԣ�
��4�����������λ��Խǿ�������Խ�ȶ�����Ϊ����õķǽ������������ڿ�����γ��ȶ�����������������ӣ�
��5��������ΪһԪ���ᣬ��ˮ��Һ���ˮ����γ���λ�������ֵ�����������ӣ�
��a�����ᾧ���д���H3BO3���ӣ����ݾ����д��ڵ���ȷ���������ͣ�
b�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬��ʯī���ƵIJ�״�ṹ��
c�����ᾧ���д���H3BO3���ӣ����ᾧ���Ƿ��Ӿ��壻
d���������ᾧ��ΪƬ��״�ṹ������
e������Bԭ�ӵļ۲���Ӷ��ж����ӻ����ͣ�
f�����ӵ��ȶ����뻯ѧ���йأ�
g�����þ�̯�����㺬1molH3BO3�ľ����е��������1mol H3BO3�ľ�������3mol�����
h���ɽṹ��֪����ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ�
�����ᣨH3BO3����һ��Ƭ��״�ṹ�������ƻ������ᾧ���ڷ���֮��������
��� �⣺��1��BԪ��Ϊ5��Ԫ�أ�ԭ�Ӻ�����5�����ӣ��������Ų�����һ��2�����ڶ���3�������Ժ�������Ų�ʽΪ��1s22s22p1��
�ʴ�Ϊ��1s22s22p1��
��2�������������ÿ����ԭ�Ӻ���4�����ۼ�������Bԭ�Ӳ���sp3�ӻ���
�ʴ�Ϊ��sp3�ӻ���
��3����Ԫ�ؾ���ȱ�����ԣ��仯���������йµ��ӶԵķ��ӻ������γ�����BF3����NH3��Ӧ����BF3•NH3��B��N֮���γ���λ����Nԭ�Ӻ��йµ��Ӷԣ����Ե�ԭ���ṩ�µ��Ӷԣ�BF3•NH3�ṹʽΪ��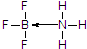 ��
��
�ʴ�Ϊ��BF3•NH3��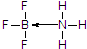 ��
��
��4��ȱ���ӻ�������к�ǿ�Ľ��ܵ��ӵ������������Ԫ���γɵ�BF3Ϊȱ���ӻ�������������γ�BF4-����Ԫ�ؾ���ȱ�����ԣ������������ڿ�����γ��ȶ��������BF4-��
�ʴ�Ϊ��BF4-��
��5��������ΪһԪ���ᣬ��ˮ��Һ���ˮ������������������γ���λ��������뷽��ʽΪ��H3BO3+H2O?[B��OH��4]-+H+��
�ʴ�Ϊ��H3BO3+H2O?[B��OH��4]-+H+��
��a�����ᾧ���д���H3BO3���ӣ��Ҹþ����д��������˵�������ɷ��ӹ��ɣ��Ƿ��Ӿ��壬ԭ�Ӿ�����ֻ�й��ۼ�����a����
b�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬���ڵġ�H3BO3����֮��ͨ�������������b��ȷ��
c�����ᾧ���д���H3BO3���ӣ����ᾧ���Ƿ��Ӿ��壬��c��ȷ��
d�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬Ƭ��״�ṹ�����л���У�����������d��ȷ��
e����B��OH��3��Ԫ�У�Bֻ�γ���3��������û�йµ��Ӷԣ����Բ�ȡsp2�ӻ�����e����
f�����ӵ��ȶ���������ڵ�B-O��H-O���ۼ��йأ��۷е�������йأ���f����
g��1����������γ���6���������ÿ�������2��������ӹ��õģ�����ƽ����3�����������1molH3BO3�ľ�������3mol�������g��ȷ��
h����ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ����Bԭ�Ӳ���8e-�ȶ��ṹ����h����
�ʴ�Ϊ��bcdg��
�۾�������������������ܽ⣬�����ƻ������ᾧ���ڷ���֮�����������Լ���ʱ��������ܽ������
�ʴ�Ϊ������ʱ���ᾧ���еĴ�������в��ֶ������£���������������������ܽ⣮
���� ���⿼�����й����֪ʶ�����ؿ���ԭ�Ӻ�������Ų����ӻ����۵�Ӧ�á����ᾧ�徧�����͵��жϡ�Ӱ������ȶ��Ե����ص�֪ʶ�㣬��Ŀ�Ѷ��еȣ�ע����ӵ��ȶ����뻯ѧ���йأ����ʵ��۷е�������йأ�ע�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壮

| A�� | pH��ͬ�Ģ�CH3COONa����NaHCO3����C6H5ONa������Һ�е�c��Na+�����ۣ��ڣ��� | |
| B�� | �����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��Na2CO3��Һ��NaHCO3��Һ��������������Һ�У�2c��OH-��-2c��H+��=3c��H2CO3��+c��HCO3-��-c��CO32-�� | |
| C�� | ��Ũ�ȡ��������Na2CO3��NaHCO3��ϣ�$\frac{c��HC{O}_{3}^{-}��}{c��{H}_{2}C{O}_{3}��}$��$\frac{c��C{O}_{3}^{2-}��}{c��HC{O}_{3}^{-}��}$ | |
| D�� | ������AgCl�ֱ���룺��5mLˮ ��10mL 0.2mol/L MgCl2 ��20mL 0.3mol/L���� ���ܽ�������c��Ag+�����٣��ڣ��� |

| A�� | ����Ӧ��H��0 | |
| B�� | n��NH3��/n��SO2����a��b��c | |
| C�� | ��ͬ�����£���������Խ��SO2��ƽ��ת����Խ�� | |
| D�� | ��ʱ����ϵ�г�ȥˮ��ƽ�ⳣ������ |
| A�� | AlCl3��Һ���ռ���Һ��Ӧ����n��OH-����n��Al3+��=7��2ʱ��2Al3++7OH-�TAl��OH��3��+AlO2-+2H2O | |
| B�� | Cl2��FeBr2��Һ��Ӧ����n��Cl2����n��FeBr2��=1��1ʱ��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl- | |
| C�� | CuCl2��Һ��NaHS��Һ��Ӧ����n��CuCl2����n��NaHS��=1��2ʱ��Cu2++2HS-�TCuS��+H2S�� | |
| D�� | Fe��ϡ���ᷴӦ����n��Fe����n��HNO3��=1��2ʱ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O |
| A�� | Ũ���� | B�� | ϡ���� | C�� | Ũ���� | D�� | ϡ���� |

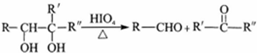
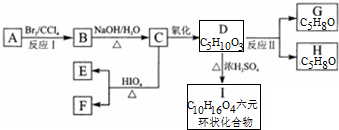
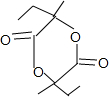 +2H2O��
+2H2O��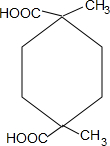 ��
��
 ]2-Na+
]2-Na+ ��
��
 ��
��