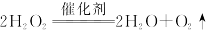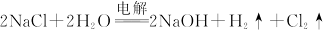��Ŀ����
��17�֣���ҵ���ƴ���ķ�������ʳ��ˮ��ͨ�백���Ͷ�����̼�����̼�����ƾ��壬�ٽ�����̼�����ƾ�����ȷֽ�ɵõ����������Ʒ��Na2CO3����������Ϊ92%~96%�����漰���Ļ�ѧ����ʽ�У�
NH3+CO2+H2O �� NH4HCO3��NH4HCO3+NaCl�����ͣ��� NaHCO3��+NH4Cl��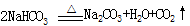 ����ش�
����ش�
��1����ҵ�ƵõĴ����г��������������Ȼ������ʣ�����Ҫԭ���� ��
��2�����мס��ҡ�������ѧ�������ⶨij��ҵ������Ʒ��Na2CO3�������������ֱ�������·�����������������������ʵ�顣
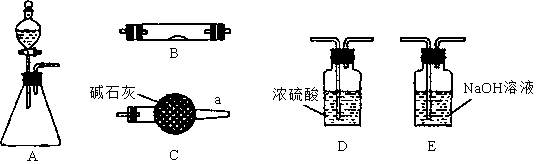
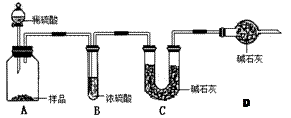
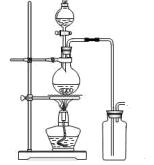
�ף��� ����ʵ����������ȡ10.0g��Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C�м�ʯ������3.52g��װ��D�м�ʯ�ҵ������� ��
�ң�ȷ��ȡ10.00g��Ʒ���� ����ʵ�����������1000mL��Һ����
ʽ�ζ�����ȡ25.00mL������ƿ�У�����2�η�̪��ָʾ������0.15mol��L��1�ı�������Һ�ζ����յ㣨�йط�ӦΪNa2CO3+HCl �� NaCl+NaHCO3�����������ƽ��ʵ����������������ƽ��ֵΪ15.00mL��
����ȷ��ȡ10.00g��Ʒ�������м�����������ᣬ��ַ�Ӧֱ����Ʒ��������ð�������ɻ����Һ�����õ����������ڸ���������ȴ�����º�������������ȡ���ȴ��������ֱ���������Ĺ���������������Ϊֹ����ʱ���ù��������Ϊ10.99g�������������������

NH3+CO2+H2O �� NH4HCO3��NH4HCO3+NaCl�����ͣ��� NaHCO3��+NH4Cl��
 ����ش�
����ش���1����ҵ�ƵõĴ����г��������������Ȼ������ʣ�����Ҫԭ���� ��
��2�����мס��ҡ�������ѧ�������ⶨij��ҵ������Ʒ��Na2CO3�������������ֱ�������·�����������������������ʵ�顣
�ף��� ����ʵ����������ȡ10.0g��Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C�м�ʯ������3.52g��װ��D�м�ʯ�ҵ������� ��
�ң�ȷ��ȡ10.00g��Ʒ���� ����ʵ�����������1000mL��Һ����
ʽ�ζ�����ȡ25.00mL������ƿ�У�����2�η�̪��ָʾ������0.15mol��L��1�ı�������Һ�ζ����յ㣨�йط�ӦΪNa2CO3+HCl �� NaCl+NaHCO3�����������ƽ��ʵ����������������ƽ��ֵΪ15.00mL��
����ȷ��ȡ10.00g��Ʒ�������м�����������ᣬ��ַ�Ӧֱ����Ʒ��������ð�������ɻ����Һ�����õ����������ڸ���������ȴ�����º�������������ȡ���ȴ��������ֱ���������Ĺ���������������Ϊֹ����ʱ���ù��������Ϊ10.99g�������������������
| ��������� ���� | ������Ʒ��̼���Ƶ��������� | ʵ���������� | ʵ��ʧ�ܵ���Ҫԭ�Խ����Ӱ�� |
| �� | | ʧ �� | |
| �� | |  �� �� �� �� | |
| �� | |  �� �� �� �� | |
��1���ڱ����Ȼ�����Һ��ͨ�백���Ͷ�����̼������ӦNH3+CO2+H2O=NH4HCO3�������˲���ˮ���Ӷ����в����Ȼ��ƾ������� ��2�֣�
��2���ף�������ƽ��ҩ�ס���ֽ����2�֣���һ����0.5�֣�������ֹ�����е�ˮ�����Ͷ�����̼����C����Сʵ����� ��2�֣�
�ң��ձ�������������ͷ�ιܡ�1000mL ������ƿ��2�֣������� ��1�֣���
��������8�֣�ÿ��2�֣�
��2���ף�������ƽ��ҩ�ס���ֽ����2�֣���һ����0.5�֣�������ֹ�����е�ˮ�����Ͷ�����̼����C����Сʵ����� ��2�֣�
�ң��ձ�������������ͷ�ιܡ�1000mL ������ƿ��2�֣������� ��1�֣���
��������8�֣�ÿ��2�֣�
| ������Ʒ��̼���Ƶ��������� | ʵ��ʧ�ܵ���Ҫԭ�Խ����Ӱ�� |
| 84.8% | ��Ӧ���ɵ�CO2���ֲ�����ƿA�У�û�б���ȫ���գ����ƫС |
| 95.4% | |
| 95.4% | |
��1����NaCl�γɱ�����Һ������NaCl����Ӧ�����ܼ�H2O�����йأ�������NaCl��Һ��ͨ��NH3��CO2������Ӧʱ������Ҫ����H2O����������NaCl���������Ӷ�����NaHCO3�У�������NaHCO3ʱ��NaCl������ʧ���γ����ʡ�
��2�����ݼײⶨԭ������Ʒ�е�Na2CO3�����ᷴӦ����CO2��H2O������װ��C���������ӵó�CO2�������Ӷ��õ�Na2CO3�������̶��õ�Na2CO3���������������ڿ������е�CO2��H2O�����װ��C������������װ��D����ֹ������CO2��H2O����װ��C������ʵ���������ҷ����ⶨԭ�����������к͵ζ�ԭ�����ⶨNa2CO3�����ģ�ȷ��ȡ��Ʒ������1000mL ����ƿ���ձ�������������ͷ�ι���������Ʒ���Ƴ���Һ��������Ʒ��Һ�ʼ��ԣ��������ü�ʽ�ζ�����ʢװ���������Ǹ�����Ʒ�е�Na2CO3�������ַ�����������������ȷ��Na2CO3�����������м�ʯ�����յ�CO2����Ϊ3.52g���ɵ�Na2CO3����Ϊ��0.08mol��106g/mol��8.48g��Na2CO3����������84.8%<92%���ⶨ���ƫ�ͣ�˵��װ���е�CO2û�б���ʯ����ȫ���ա��ҷ����У�n(Na2CO3)��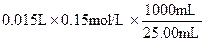 ��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������
��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������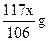 �����У�10.00��x ��
�����У�10.00��x ��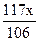 ��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��
��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��
��2�����ݼײⶨԭ������Ʒ�е�Na2CO3�����ᷴӦ����CO2��H2O������װ��C���������ӵó�CO2�������Ӷ��õ�Na2CO3�������̶��õ�Na2CO3���������������ڿ������е�CO2��H2O�����װ��C������������װ��D����ֹ������CO2��H2O����װ��C������ʵ���������ҷ����ⶨԭ�����������к͵ζ�ԭ�����ⶨNa2CO3�����ģ�ȷ��ȡ��Ʒ������1000mL ����ƿ���ձ�������������ͷ�ι���������Ʒ���Ƴ���Һ��������Ʒ��Һ�ʼ��ԣ��������ü�ʽ�ζ�����ʢװ���������Ǹ�����Ʒ�е�Na2CO3�������ַ�����������������ȷ��Na2CO3�����������м�ʯ�����յ�CO2����Ϊ3.52g���ɵ�Na2CO3����Ϊ��0.08mol��106g/mol��8.48g��Na2CO3����������84.8%<92%���ⶨ���ƫ�ͣ�˵��װ���е�CO2û�б���ʯ����ȫ���ա��ҷ����У�n(Na2CO3)��
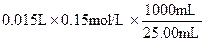 ��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������
��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������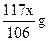 �����У�10.00��x ��
�����У�10.00��x ��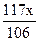 ��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��
��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��
��ϰ��ϵ�д�
�����Ŀ
 H++HCO3- Ka1 =4��45��10-7
H++HCO3- Ka1 =4��45��10-7