��Ŀ����
18���۱�ϩ���ƣ�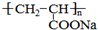 ����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ�������ͼ��
����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ�������ͼ��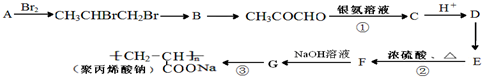
��1��A������Ϊ��ϩ��B�й����ŵ��������ǻ���
��2������E�Ľṹ��ʽ��CH3CH��OH��COOH��
��3����Ӧ�ڡ��۵ķ�Ӧ���ͷֱ�Ϊ��ȥ��Ӧ���Ӿ۷�Ӧ��
��4����Ӧ�ٷ����Ļ�ѧ����ʽΪ
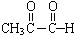 +2Ag��NH3��2OH$\stackrel{��}{��}$
+2Ag��NH3��2OH$\stackrel{��}{��}$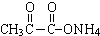 +3NH3+2Ag��+H2O��
+3NH3+2Ag��+H2O����5��д����������������F��ͬ���칹�壨������˳���칹���Ľṹ��ʽHCOOCH=CH2��
a����״����
b��������������ͭ�ڼ��������·�Ӧ���ɺ�ɫ����
c����NaOH��Һ��Ϻ�����̪�����ȣ���Һ��ɫ��dz��
���� A���巢���ӳɷ�Ӧ�IJ�����1��2-������飬����A�DZ�ϩ�����ݲ���CH3COCHO������֪��B��1��2-�������ˮ��IJ���õ��˺������ִ��ǻ����л����ʣ�B��CH3CH��OH��CH2OH��ȩ���Ժ�������Һ����������Ӧ������C��CH3COCOONH4���ữ��õ�DΪ��CH3COCOOH�����ݼӾ۲���۱�ϩ���ƣ����Եó�G�DZ�ϩ���ƣ���F�DZ�ϩ�ᣬ����E��CH3COCOOH�������ӳ��Ժ�õ��Ĵ�����Ϊ��CH3CH��OH��COOH���ô����Է�����ȥ��Ӧ
��1������A����ӳɵIJ�����ȷ��A�Ľṹ��ʽ�����ݴ��Ļ�ѧ�������ش�
��2�����Ц�-H�Ĵ����Է�����ȥ��Ӧ����̼̼˫����
��3���������ʵ������Լ�������ת����ȷ����Ӧ���ͣ�
��4��ȩ���Ժ�������Һ����������Ӧ����������Ρ�ˮ�����Լ�������
��5��������������ͭ�ڼ��������·�Ӧ���ɺ�ɫ���������ʺ���ȩ���������Ȼ������ʿ��Ժ��������Ʒ����кͷ�Ӧ��
��� �⣺A���巢���ӳɷ�Ӧ�IJ�����1��2-������飬����A�DZ�ϩ�����ݲ���CH3COCHO������֪��B��1��2-�������ˮ��IJ���õ��˺������ִ��ǻ����л����ʣ�B��CH3CH��OH��CH2OH��ȩ���Ժ�������Һ����������Ӧ������C��CH3COCOONH4���ữ��õ�DΪ��CH3COCOOH�����ݼӾ۲���۱�ϩ���ƣ����Եó�G�DZ�ϩ���ƣ���F�DZ�ϩ�ᣬ����E��CH3COCOOH�������ӳ��Ժ�õ��Ĵ�����Ϊ��CH3CH��OH��COOH���ô����Է�����ȥ��Ӧ��
��1��A�������DZ�ϩ��B��CH3CH��OH��CH2OH�����еĹ����ŵ��������ǻ����ʴ�Ϊ����ϩ���ǻ���
��2��E�Ľṹ��ʽ��CH3CH��OH��COOH���ʴ�Ϊ��CH3CH��OH��COOH��
��3��CH3CH��OH��COOHת��ΪCH2�TCHCOOH�ķ�Ӧ����ȥ��Ӧ����ϩ����ת��Ϊ�۱�ϩ���Ƶķ�Ӧ���ڼӾ۷�Ӧ���ʴ�Ϊ����ȥ��Ӧ���Ӿ۷�Ӧ��
��4��CH3COCHO����ȩ�����ʣ����Է���������Ӧ��ԭ������ʽΪ��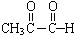 +2Ag��NH3��2OH$\stackrel{��}{��}$
+2Ag��NH3��2OH$\stackrel{��}{��}$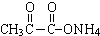 +3NH3+2Ag��+H2O��
+3NH3+2Ag��+H2O��
�ʴ�Ϊ��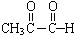 +2Ag��NH3��2OH$\stackrel{��}{��}$
+2Ag��NH3��2OH$\stackrel{��}{��}$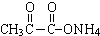 +3NH3+2Ag��+H2O��
+3NH3+2Ag��+H2O��
��5��FΪ��ϩ�ᣬ���ķ���ʽΪ��C3H4O2������ͬ���칹���У���������������ͭ�ڼ��������·�Ӧ���ɺ�ɫ������˵������ȩ������NaOH��Һ��Ϻ�����̪�����ȣ���Һ��ɫ��dz��֤�����Ȼ������Խṹ��ʽΪ��HCOOCH=CH2���ʴ�Ϊ��HCOOCH=CH2��
���� ���⿼���л���ĺϳ����ƶϡ������š�ͬ���칹�塢�л���Ӧ���͵ȣ���ȷ�ϳ��з�Ӧ�������������ŵı仯���ɽ����Ŀ�Ѷ��еȣ�

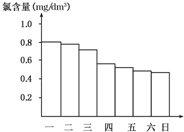 ijУ��ѧ�о���ѧϰС����ճ������е���������;�����˵��飬�ڵ���ij������Ӿ���ļ���ˮ�������ʱ����֪����Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ��������������ͼ�Ǹ�С��ÿ��19��00ʱ�ⶨ����Ӿ����ˮ�ĺ��������ɴ��Ʋ��������ȡ�����ǿ������ܵ�һ���ǣ�������
ijУ��ѧ�о���ѧϰС����ճ������е���������;�����˵��飬�ڵ���ij������Ӿ���ļ���ˮ�������ʱ����֪����Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ��������������ͼ�Ǹ�С��ÿ��19��00ʱ�ⶨ����Ӿ����ˮ�ĺ��������ɴ��Ʋ��������ȡ�����ǿ������ܵ�һ���ǣ�������| A�� | ��һ | B�� | �ܶ� | C�� | ���� | D�� | ���� |
| A�� | �����Ԫ��ԭ������㶼ֻ��1������ | |
| B�� | ��Li��Na��K��Rb�������۷е����ߣ��ܶ����� | |
| C�� | ���������ԣ�F2��Cl2��Br2��I2 | |
| D�� | �⻯���ȶ��ԣ�HF��HCl��HBr��HI |
| ��֪ | ���� | |
| A | ��Fe����CuSO4��Һ�У� Fe+Cu2+�TCu+Fe2+ | ��Na���뵽CuSO4��Һ�У� 2Na+Cu2+�TCu+2Na+ |
| B | ��ϡ������Һ�м���NaOH��Һ�����ԣ�H++OH-�TH2O | ��ϡH2SO4��Һ�м���Ba��OH��2��Һ�����ԣ�H++OH-�TH2O |
| C | ����ˮ����ƿ���ڼ�������ϡ������Һ�� CaCO3+2H+�TCa2++CO2��+H2O | ����ˮ����ƿ���ڼ�������������Һ�� CaCO3+2H+�TCa2++CO2��+H2O |
| D | ��Ca��OH��2��Һ��ͨ�����CO2�� CO2+OH-�THCO3- | ��Ca��OH��2��Һ��ͨ�����SO2�� SO2+OH-�THSO3- |
| A�� | A | B�� | B | C�� | C | D�� | D |
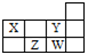
| A�� | 1��37Wԭ���У�����������������3�� | |
| B�� | Y��W����ͬһ���壬������ͬ�����̬ | |
| C�� | Z�γɼ������Ӱ뾶С��W�γɼ������Ӱ뾶 | |
| D�� | X��Y��Z��Wÿ������Ԫ�ؼ�����γɵ�������ȵ��⻯�� |
| A�� | 3 mol/L | B�� | 0.3molL/��L•s�� | C�� | 0.6 molL/��L•s�� | D�� | 0.1 molL/��L•s�� |
| A�� | �������м�����������Һ��Cl-+Ag+�TAgCl�� | |
| B�� | ����������������ˮ��Һ���ȣ�CH3CH2Br+NaOH$��_{��}^{H_{2}O}$CH2�TCH2��+NaBr+H2O | |
| C�� | ��ϩ�ۺϣ�nCH2�TCHCH3$��_{��}^{����}$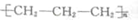 | |
| D�� | ��������Һ��ͨ������������̼��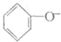 +CO2+H2O�� +CO2+H2O��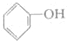 +HCO3- +HCO3- |
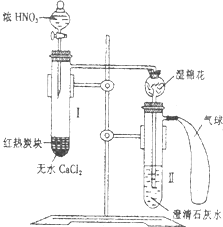 ijУΪ�˸Ľ�̼��HN03��Ӧ��ʵ�飬�������ͼ��ʾ��װ�ã���Ԥ��ʢ����ˮCaCl2���Թ�I��Ȼ��Ͷ�뼸С����ȵ�̿�飬�ٻ�������̿���ϵμ�ŨHN03����Ӧ�����������У�
ijУΪ�˸Ľ�̼��HN03��Ӧ��ʵ�飬�������ͼ��ʾ��װ�ã���Ԥ��ʢ����ˮCaCl2���Թ�I��Ȼ��Ͷ�뼸С����ȵ�̿�飬�ٻ�������̿���ϵμ�ŨHN03����Ӧ�����������У�