��Ŀ����
2�� �����ᣨH3PO3������������������Ǧ������ȵķ����Լ���
�����ᣨH3PO3������������������Ǧ������ȵķ����Լ�����1��������Ϊ��Ԫ���ᣬ������ˮ��Һ�еĵ��뷽��ʽ�ɱ�ʾΪH3PO3?H++H2PO3-��H2PO3-?H++HPO3-������������ƣ�Na2HPO3���������Σ�����Ρ�����ʽ�Ρ�������֪���������ƽ�ⳣ��K1=1.6��10-2��K2=7��10-7������ĵ���ƽ�ⳣ��K=1.8��10-5������������Һ���������Һ��Ӧ�����ӷ���ʽΪH3PO3+CH3COO-�TH2PO3-+CH3COOH��
��2����������ͨ������±Ƚ��ȶ����ڸ���180��ʱ�ᷢ���ֽ⣬�����������ʣ���֪���е�һ����PH3����÷�Ӧ�Ļ�ѧ����ʽΪ4H3PO3�TPH3+3H3PO4��
��3������������Һ�пɽ�Ag+��ԭ���йط�Ӧ�����ӷ���ʽΪ2Ag++H3PO3+H2O�TH3PO4+2Ag+2H+����������ʹ��ˮ��ɫ���йط�Ӧ�Ļ�ѧ����ʽΪI2+H3PO3+H2O�T2 HI+H3PO4��
��4�����Na2HPO3��ҺҲ�ɵõ������ᣬ��װ��ʾ��ͼ��ͼ��ʾ��
�������ĵ缫��ӦʽΪ2H++2e-=H2����
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪHPO32-+2H+=H3PO3��
���� ��1����Ԫ����ĵ����Ƿֲ����еģ�������Ϊ��Ԫ���ᣬ������������ƣ�Na2HPO3�������Ե���������ӣ���ǿ�������������Ӧ����ʽ��
��2����������ԭ��Ӧ�е�ʧ�����غ���д��ѧ����ʽ��
��3���������Ag+���ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬAg+����ԭΪAg���ⱻ��ԭ��������ᣬ�ݴ�д����Ӧ����ʽ��
��4���������ϵõ��ӷ�����ԭ��Ӧ��
�ڲ�Ʒ����HPO32-�������ӽ�� ���������ᣮ
��� �⣺��1�������ᣨH3PO3��Ϊ��Ԫ���ᣬ����ˮ��Һ�еĵ��뷽��ʽ�ɱ�ʾΪ��H3PO3?H++H2PO3-��H2PO3-?H++HPO3-��������Ϊ��Ԫ���ᣬ������������ƣ�Na2HPO3�������Ե���������ӣ�Ϊ���Σ��ݵ���ƽ�ⳣ������������ǿ��˳��Ϊ��H3PO3��CH3COOH��H2PO3-��������������Һ���������Һ��Ӧ�����ӷ���ʽΪH3PO3+CH3COO-�TH2PO3-+CH3COOH��
�ʴ�Ϊ��H3PO3?H++H2PO3-��H2PO3-?H++HPO3-�����Σ�H3PO3+CH3COO-�TH2PO3-+CH3COOH��
��2��H3PO3��PΪ+3�ۣ��ֽ����ɵ�PH3��PΪ-3�ۣ�����һ�ַֽ������P�Ļ��ϼ�Ӧ�����ߣ���ֽ�Ļ�ѧ����ʽΪ��4H3PO3�TPH3+3H3PO4��
�ʴ�Ϊ��4H3PO3�TPH3+3H3PO4��
��3���������Ag+����������ԭ��Ӧ������������ԭ���������������ᣬAg+����ԭΪAg����Ӧ�����ӷ���ʽΪ��2Ag++H3PO3+H2O�TH3PO4+2Ag+2H+��������͵ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬ�ⱻ��ԭ��������ᣬ��Ӧ����ʽΪ��I2+H3PO3+H2O�T2 HI+H3PO4��
�ʴ�Ϊ��2Ag++H3PO3+H2O�TH3PO4+2Ag+2H+��I2+H3PO3+H2O�T2 HI+H3PO4��
��4���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪHPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��
���� ���⿼���Ϊ�ۺϣ��漰ǿ�������ᡢ������ԭ��Ӧ���缫��Ӧʽ����д��֪ʶ�㣬�缫��Ӧʽ����д�����ӷ���ʽ��д�Ǹ߿��ȵ㣬Ӧ�ص����գ��Ѷ��еȣ�

| A�� | 8�� | B�� | 9�� | C�� | 10�� | D�� | 7�� |
 �����£���0.1mol/L��ˮ�ֱ�ζ�20.0mL��0.1mol/L������ʹ��ᣬ������ͼ��ʾ������˵����ȷ�ģ�������
�����£���0.1mol/L��ˮ�ֱ�ζ�20.0mL��0.1mol/L������ʹ��ᣬ������ͼ��ʾ������˵����ȷ�ģ�������| A�� | I���߱�ʾ���ǵζ���������� | |
| B�� | x=20 | |
| C�� | �ζ�������$\frac{c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$��ֵ��С | |
| D�� | ��I���ߺ�II����pH��Ϊ7ʱ��һ��Һ�е�c��Cl-��������һ��Һ�е�c��CH3COO-�� |

��֪��AlO2-+HCO3-+H2O=Al��OH��3��+CO32-
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ���ǣ�������
| A�� | ����2�����ڿ����У����������ӣ������п϶�����SO32- | |
| B�� | ������Һ�м����Թ�����NH4C1��Һ�ܵõ������1�ɷ���ͬ������ | |
| C�� | �������̲��裨1����2����3����˳���Ϊ��3����2����1�������ܻ�����ͬ��ʵ������ | |
| D�� | ������Һ�п϶�����A102-��Cl-������Na+��K+���ٴ���1�� |
| A�� | 1mol�ױ���C-H������ĿΪ6NA | |
| B�� | ��Ӧ5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O������28gN2ʱ��ת�Ƶ�����ĿΪ15NA | |
| C�� | 1molFeCl3��ˮ��Ӧ��ȫת��Ϊ������������������ӵ���ĿΪNA | |
| D�� | ����4.6g��Ԫ�صĹ������ƺ������ƵĻ�����У�������������Ϊ0.3NA |
| ���� | H2SO3 | HClO | H2CO3 |
| Ka1 | 1.54��10-2 | 2.95��10-8 | 4.30��10-7 |
| Ka2 | 1.02��10-7 | - | 5.61��10-11 |
| A�� | NaClO��Һ��ͨ������CO2��ClO-+H2O+CO2�THCO3-+HClO | |
| B�� | ��ˮ�м�������NaCO3��ĩ��HCO3-+H+�TH2O+CO2�� | |
| C�� | NaClO��Һ��ͨ������SO2��2ClO-+H2O+SO2�TSO32-+2HClO | |
| D�� | Na2CO2��Һ�л���ͨ������SO2��2CO32-+H2O+SO2�TSO32-+2HCO3- |
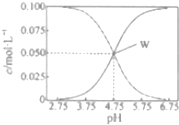 25��ʱ����c��CH2COOH��+c��CH2COO-��=0.1mol•L-1��һ����ᡢ�����ƻ����Һ����Һ��c��CH2COOH����c��CH2COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ����������ȷ���ǣ�������
25��ʱ����c��CH2COOH��+c��CH2COO-��=0.1mol•L-1��һ����ᡢ�����ƻ����Һ����Һ��c��CH2COOH����c��CH2COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ����������ȷ���ǣ�������| A�� | 25��ʱ������ĵ��볣��Ka=1��10-4.75 | |
| B�� | W������ʾ����Һ�У�c��Na+��+c��H+��=c��CH3COOH��+c��OH-�� | |
| C�� | pH=5.5����Һ�У�c��CH3COOH����c��CH3COO-����c��H+����c��OH-�� | |
| D�� | pH=3.5����Һ�У�c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol•L-1 |
| A�� | ����Һ�У�Na+��Fe3+��NO3-��Cl-���ܴ������� | |
| B�� | ����ˮ��Ӧ�����ӷ���ʽ��Br2+SO32-+H2O=2H++2Br-+SO42- | |
| C�� | ��Ba��OH��2��Һ���ȷ�Ӧ�����ӷ���ʽ��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| D�� | 1L0.1mol•L-1����Һ�������������ַ�Ӧ������2.24L����״����SO2���� |
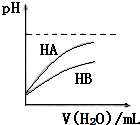
| A�� | �����ʵ���Ũ��Ϊ10-3mol/L�Ĵ����pH=11��NaOH��Һ�������Ϻ���Һ�Լ��� | |
| B�� | ��ͼ�ɱ�ʾ�����£�ϡ��HA��HB�������ϡ��Һʱ��ҺpH���ˮ���ı仯����ͬŨ�ȵ�NaA��Һ��pH����NaB��Һ | |
| C�� | 25��ʱ��pH=2��1.0 L ������Һ��ˮ�������H+����ĿΪ10-12NA | |
| D�� | ij���ȷ�Ӧ���Է����У���÷�Ӧһ���������ķ�Ӧ |