��Ŀ����
17����NA��ʾ����٤��������ֵ������˵����ȷ���ǣ�������| A�� | 1mol�ױ���C-H������ĿΪ6NA | |
| B�� | ��Ӧ5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O������28gN2ʱ��ת�Ƶ�����ĿΪ15NA | |
| C�� | 1molFeCl3��ˮ��Ӧ��ȫת��Ϊ������������������ӵ���ĿΪNA | |
| D�� | ����4.6g��Ԫ�صĹ������ƺ������ƵĻ�����У�������������Ϊ0.3NA |
���� A���ױ������к���8��̼�����1mol�����к���8mol̼�����
B���÷�Ӧ�У�笠�������Nԭ����ȫת���ɵ������ݴ��ж�ת�Ƶĵ�������
C��������������Ϊ���������ľۼ��壬����������������������Ŀ��
D.4.6g�����ӵ����ʵ���Ϊ0.2mol�������ƺ��������ж�����2�������ӡ�1�������ӣ��ݴ˼��㺬�е�����������
��� �⣺A.1mol�ױ��к���8mol̼����������ܹ���C-H������ĿΪ8NA����A����
B����Ӧ5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O�У�����ͬ��Ԫ�ػ��ϼ����м俿£ԭ��笠������е�Ԫ����ȫת���ɵ���������4mol����ת�Ƶĵ���Ϊ��5mol��3=15mol��28g���������ʵ���Ϊ1mol��������28gN2ʱ��ת����$\frac{15}{4}$mol=3.75mol���ӣ�ת�Ƶ�����ĿΪ3.75NA����B����
C������������������Ϊ���������ľۼ��壬�������㽺���н�������Ŀ����C����
D.4.6g�����ӵ����ʵ���Ϊ��$\frac{4.6g}{23g/mol}$=0.2mol��1mol�����ƺ��������ж�����2mol�����ӣ��������ƺ������Ƶ����ʵ���Ϊ0.1mol��0.1mol������к���0.2mol�����ӡ������������Ӻ��������ӵ������ʵ���Ϊ0.1mol���ܹ�����0.3mol���ӣ�������������Ϊ0.3NA����D��ȷ��
��ѡD��
���� ���⿼�鰢���ӵ��������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�ע�����պ������ʵ���Ϊ���ĵĸ���ѧ���밢���ӵ������Ĺ�ϵ��ȷŪ����ӡ�ԭ�ӡ�ԭ�Ӻ����������Ӽ�������ӵĹ��ɹ�ϵ������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | CH4��Cl2���շ���ȡ����Ӧ | |
| B�� | 1��3-����ϩ������ʵ������巢����Ӧ | |
| C�� | ��ϩ���Ȼ���ӳ� | |
| D�� | �Ҵ����� |
| A�� | �ϳɰ���Ӧ�ڵ������ܹ��Է����У�����Ϊ��Ӧ�����֮�ʹ������������֮�� | |
| B�� | Ԫ�ط����ǿ���ȷ���������Ƿ���C��H��O��N��S��Cl��Br��Ԫ�أ�ԭ�����չ�����ȷ�������к�����Щ����Ԫ�� | |
| C�� | ��������Ԫ���������ڹ���Ԫ����Ѱ�Ҹ������������Ĵ������Խ��ͻ�ѧ��Ӧ�Ļ�ܣ��Ӷ��ܺõĽ���Ч�� | |
| D�� | ���߷ֱ���ӫ�������ܹ��۲쵽���׳߶ȵ����ʣ��������Ի�õ�������Һ�еķ���ͼ�� |
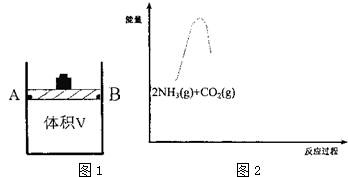
��C02��g��+2NH3��g��?NH2COONH4��s����H1
��NH2COONH4��s��?CO��NH2��2��s��+H20��g����H2��0 ��ش��������⣺
��1���о���Ӧ�ٵ�ƽ�ⳣ����K�����¶ȣ�T���Ĺ�ϵ����ͼ1��ʾ�����H1���� ��ѡ���������������
��2��������ɱ䣨����������֮���Ħ�������Բ��ƣ����ܱ�������ͼ2��ʾ���ֽ�3molNH3��2molC02���� �����У��ƶ����������VΪ3L����í���̶���A��B�㣬�����ϳ����ص��ܷ�Ӧ���£�
C02��g��+2NH3��g��?CO��NH2��2��s��+H20��g���ⶨ��ͬ��������ͬʱ����ڵ�C02��ת���ʣ��õ��������ݣ�
| CO2ת���� T���棩 | 10min | 20min | 30min | 40min |
| T1 | 30% | 65% | 75% | 75% |
| T | 45% | 50% | a1 | a2 |
�ڸ����ϱ����ݣ���Ƚ�T1��T2��ѡ���������������T2���£���30minʱ��a150%�����¶��µĻ�ѧƽ�ⳣ��Ϊ9��
��T2���£���40minʱ����ȥí���������ܷ������ã�����û�з����ƶ�������������ͨ��3molC02����ʱv������= v���棩��ѡ����������������жϵ�������T2���£�ͨ��3molC02�������������Ϊԭ����2����Qc=9=K��ƽ�ⲻ�ƶ���
��3������ͼ��2�������ϳɰ��ܷ�Ӧ2NH3��g��+C02��g��?C0��NH2��2��s��+H2O��g�������������仯ͼ���������м���NH2COONH4��s�����������CO��NH2��2��s��+H20��g������
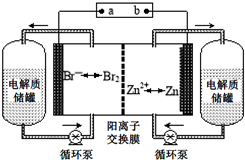 п��Һ�������һ�����͵绯ѧ����װ�ã���ͼ��ʾ�������ҺΪ�廯пˮ��Һ���������Һ�ڵ���ʴ��͵�ؼ䲻��ѭ��������˵������ȷ���ǣ�������
п��Һ�������һ�����͵绯ѧ����װ�ã���ͼ��ʾ�������ҺΪ�廯пˮ��Һ���������Һ�ڵ���ʴ��͵�ؼ䲻��ѭ��������˵������ȷ���ǣ�������| A�� | �ŵ�ʱ������ʴ����е�������Ũ������ | |
| B�� | ���ʱ�缫a���ӵ�Դ�ĸ��� | |
| C�� | �ŵ�ʱ�����ĵ缫��ӦʽΪZn-2e-�TZn2+ | |
| D�� | �����ӽ���Ĥ����ֹBr2��Znֱ�ӷ�����Ӧ |
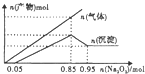
| A�� | �����Һ��m��Al3+��Ϊ5.4g | B�� | �����Һ��c��Mg2+��Ϊ0.5mol/L | ||
| C�� | �����Һ��pH=2 | D�� | �����Һ��c��Cl-��Ϊ1.7mol/L |

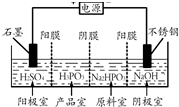 �����ᣨH3PO3������������������Ǧ������ȵķ����Լ���
�����ᣨH3PO3������������������Ǧ������ȵķ����Լ���