��Ŀ����
7����������ĵ���ƽ�ⳣ�����±���| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | K=1.77��10-4 | K=4.9��10-10 | K1=4.3��10-7��K2=5.6��10-11 |
| A�� | ��NaHCO3��Һ�У�c��OH-��-c��H+��=c��H2CO3��-c��CO32-�� | |
| B�� | ��NaCN��Һ��ͨ������CO2�����ӷ���ʽ��2CN-+H2O+CO2=2HCN+CO32- | |
| C�� | �к͵��������pH��HCOOH��HCN����NaOH����ǰ�ߴ��ں��� | |
| D�� | �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��NaHCO3����pH��NaCN����pH��HCOONa�� |
���� ����ĵ���ƽ�ⳣ��Խ��������Խǿ����pH��������Һ������Խǿ���������ʵ���Ũ��ԽС�����������ˮ��̶�ԽС�����ǿ���ܺ������η�Ӧ��ȡ����������
��� �⣺A�����ݵ���غ㣬c��HCO3-��+c��OH-��+2c��CO32-��=c��Na+��+c��H+�����������غ�c��HCO3-��+c��H2CO3��+c��CO32-��=c��Na+�����õ���غ�-�����غ�ɵ�c��OH-��-c��H+��=c��H2CO3��-c��CO32-������A��ȷ��
B��������ǿ������˳��Ϊ��H2CO3��HCN��HCO3-������ǿ���������ԭ����֪��CN-+H2O+CO2=HCN+HCO3-����B����
C��HCOOH��HCN����һԪ�ᣬ�кͼ��������ͬ����C����
D������Խ���������ˮ��̶�Խ�����Ե����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��NaCN����pH��NaHCO3����pH��HCOONa������D����
��ѡA��
���� ���⿼��������ʵĵ��룬����ƽ�ⳣ��ȷ������ǿ�����Ӷ�ȷ����֮���ת������ϵ���غ�����������Ѷ��еȣ�

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
17�������ѧ�ҳɹ��Ƴ���һ�����͵�̼��������û����ᄃ����ÿ��̼ԭ�Ӿ����ĸ����۵�������ԭ�ӽ��Ϊһ�ֿռ���״��������չ�ṹ�����жԸþ�������������ǣ�������
| A�� | �þ���������ԭ�Ӿ��� | |
| B�� | �þ�����̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ1��2 | |
| C�� | ������̼ԭ������C-O��ѧ����֮��Ϊ1��2 | |
| D�� | ����Ŀռ���С������6��ԭ�ӹ��� |
15��ʯ��������Һ�д�������ƽ����Ca��OH��2���̣�?Ca2++2OH-һ�����Ĵ�����Һ�м���������ʯ�ң�ά���¶Ȳ��䣬����˵������ȷ���ǣ�������
| A�� | ��Һ��Ca2+��Ŀ���� | B�� | c��Ca2+������ | ||
| C�� | ��Һ��c��OH-������ | D�� | ��Һ��OH-��Ŀ���� |
2����������105B��115B����ͬλ��ԭ�ӹ��ɣ���֪5.4g������ȫ��ת����B2H6�����飩����ʱ���ɵñ�״����5.6L���飬������˵����ȷ���ǣ�������
| A�� | ������105B��115B����ͬλ��ԭ�ӵ�������Ϊ1��4 | |
| B�� | 5.4 g�þ�������������Ϊ2.9 mol | |
| C�� | �������ķֱ���105B��115B���ɵľ���������������֮��Ϊ6��5 | |
| D�� | ��̼ԭ������Ϊw g����105Bԭ�ӵ�����Ϊ10w g |
 3-������
3-������ 2��2��3-��������
2��2��3-��������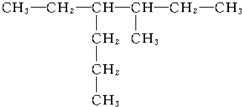 3-��-4-�һ�����
3-��-4-�һ����� 2��5-����-3-�һ�����
2��5-����-3-�һ����� 3��4-����-5-�һ�����
3��4-����-5-�һ����� 3-��-4-�һ����飮
3-��-4-�һ����飮