��Ŀ����
9�����������Ҫ����;��ij��������ͼ2ʾ�Ĺ��������������ᣮ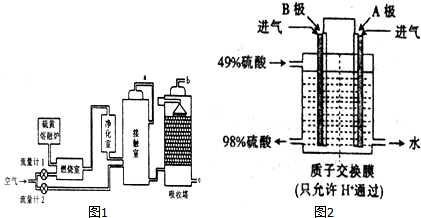
��ش��������⣺
��1������������Ϊԭ�ϵ�����������ȣ��ù��յ��ص���A��
A������������ B�����������ת�������
C�������ķ������� D������Ҫʹ�ô���
��2������a�ijɷ��Ƕ��������������
��3������Ӵ��ҵ����壬���Ⱦ��������ҵ�ԭ���Ƿ�ֹ��������еĻҳ���ˮ�������ڽӴ����е��´����ж���
��������������SO3������������98.3%Ũ���ᣮ
����SO2��O2Ϊԭ�ϣ����õ绯ѧԭ��Ҳ�����Ʊ����ᣬװ����ͼ2�缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���B���ĵ缫��ӦʽΪSO2-2e-+2H2O�TSO42-+4H+��
���������ų���β�����ð�ˮ���գ��õ����M������Ũ���� ����M���õ����{Ũ�ȵ�SO2��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2ʱ������Ӧ�����ӷ���ʽ��SO2-2e-+2H2O�TSO42-+4H+��
��Ϊȷ���������M�ijɷ֣�����ͬ��������ηֱ����50.00ml��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩����ñ�״����NH3����������
| ������/g | 2.15 | 4.30 | 6.45 |
| NH3�����/mL | 672 | 1344 | 1344 |
���� ��1��A��������������������Ҫ����O2��
B��ԭ��ѡ����SO2��ת�����أ�
C����������Ϊԭ�ϲ����ķ����϶࣬����������ͬ��
D����SO2��ȡSO3�Ĺ����ж���Ҫʹ�ô�����
��2������������������Ӧ������������Ϊ���淴Ӧ�����ܽ��е��ף�
��3���ٽӴ����о���������Է�ֹ�����ж���
�����������������յķ���������Ũ�������գ���ֹ�γ���������ֹ���շ����жϣ�
�۱���ΪSO2��O2��Ӧ����SO3��SO3����ˮ�����������ᣬ��������ij����ж�����������������������Ӧ������������ԭ��Ӧ��ԭ��طŵ�ʱ���������Һ���������������ƶ���
�����������������Ƶ����ʵ�����ͬ������1�����ɵ�������������鶼�٣��Դ��жϷ�Ӧ�Ĺ��������ݵ�1�����ݣ������۵ķ����ж����ʵijɷ֣����������狀���������淋����ʵ������������������ʵ�����ʽ��������ߵ����ʵ�����
��� �⣺��1��A��������������������Ҫ����O2����A��ȷ��
B��ԭ��ѡ����SO2��ת�����أ���B����
C����������Ϊԭ�ϲ����ķ����϶࣬����������ͬ����C����
D����SO2��ȡSO3�Ĺ����ж���Ҫʹ�ô�������D����
��ѡ��A��
��2������������������Ӧ������������Ϊ���淴Ӧ�����ܽ��е��ף�δ��Ӧ�Ķ���������������Լ���ѭ��ʹ�ã�
�ʴ�Ϊ�����������������
��3���ٽӴ����о���������Է�ֹ�����ж���
�ʴ�Ϊ����ֹ��������еĻҳ���ˮ�������ڽӴ����е��´����ж���
������������98.3%��H2SO4������SO3��ԭ������ˮ�������γ�������ֹ������������գ�
�ʴ�Ϊ��98.3%Ũ���
�۸�ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ�������Ͷ�ŵ������������������������õ��Ӻ������ӷ�Ӧ����ˮ�����������ˮ�ij��ڷ���֪��B���Ǹ�����A��������������B���ϵĵ缫��ӦʽΪ��SO2-2e-+2H2O�TSO42-+4H+��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
�����������������Ƶ����ʵ�����ͬ������1�����ɵ�������������鶼�٣����Ե�1���������ƹ�����1���а�ɫ���巴Ӧ��ȫ��1�鷴Ӧ���ɵİ���Ϊ��n��NH3��=$\frac{0.672L}{22.4L/mol}$=0.03mol��
����ȫΪ��NH4��2SO3�����ɵ�NH3Ϊ��n��NH3��=$\frac{2.15g}{116g/mol}$��2=0.037mol��0.03mol��
��AȫΪNH4HSO3�����ɵ�NH3Ϊ��n��NH3��=$\frac{2.15g}{99g/mol}$=0.022mol��0.03mol��
���Եõ�����Ϊ��NH4HSO3�ͣ�NH4��2SO3����
�裨NH4��2SO3���ʵ���Ϊxmol��NH4HSO3���ʵ���Ϊymol��
���ݵ�ԭ�Ӹ����غ�ã�2x+y=0.03��
���������غ�ã�116x+99y=2.15��
��ã�x=y=0.01mol�����Զ������ʵ���֮��Ϊ��1��1��
�ʴ�Ϊ��NH4HSO3�ͣ�NH4��2SO3��1��1��
���� �����Թ�ҵ�Ʊ�������Ĺ�������Ϊ���忼���˽Ӵ����Ʊ������ԭ�����漰���ʵ����ʡ��缫��Ӧʽ����д�������ļ��㣬ע��Ա������ݵķ����ǽ���ؼ�������ѧ�������ݷ���������������ע�����۷���Ӧ�ã�

| A�� | ��10mL��Ͳ��ȡ7.36mLNaOH ��Һ | |
| B�� | ��������ƽ��ȡ6.85gʳ�� | |
| C�� | �ñ�������ζ�δ֪Ũ�ȵ�NaOH ��Һ����ȥ����21.10mL | |
| D�� | �ù㷺pH ��ֽ���ij��ҺpH Ϊ5.5 |
| A�� | ��ˮ�����ʵ���Ũ�ȵ�������������Һ | |
| B�� | ����ˮ���ϡ��10����pH��11 | |
| C�� | �������ˮ������������Һ�������Ȼ�����Һ��Ӧ����ˮ�����ij����� | |
| D�� | ��������İ�ˮ������������ϡ������ȫ�кͣ���������������ͬ |
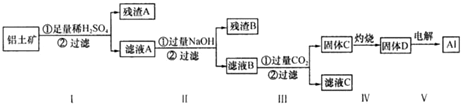
����˵������ȷ���ǣ�������
| A�� | ��ҺA �����ھ�ˮ���侻ˮԭ��ΪAl3++3H2O�TAl��OH��3+3H+ | |
| B�� | ����C������D �IJ�����������Ҫ�����Ǿƾ��ơ����������� | |
| C�� | ��ҺC �д������¹�ϵ��c��Na+��=c��SO42-��+2 c��CO32-��+c��HCO3-��+c��OH-��-c��H+�� | |
| D�� | ����100 mL ��ҺB �м���1 mol•L-1HCl 200 mL�������ﵽ���������Ϊ11.7 g������ҺB ��c��Na+����С2 mol•L |
| A�� | 1��1��1 | B�� | 1��1��2 | C�� | 1��2��3 | D�� | 2��2��3 |
| A�� | �������Ծ�ˮ��Al3++3H2O=Al��OH��3�����壩��+3H+ | |
| B�� | ����С�մ���Һ����Ba��OH��2��Һ�У�HCO-3+Ba2++OH-=BaCO3��+H2O | |
| C�� | ���⻯�Ʒ���ˮ�⣺HS-+H2O?H3O++S2- | |
| D�� | ������������ϡ���FeO+2H+=Fe2++H2O |
 �����к��еĻ�ѧ������Ϊ���Ӽ����ۼ���
�����к��еĻ�ѧ������Ϊ���Ӽ����ۼ���