��Ŀ����
4��X��Y��Z��W��R��5�ֶ�����Ԫ�أ���ԭ��������������X�γɵĵ������ܶ���С�����ʣ�Yԭ����������������������$\frac{3}{4}$����Z��W��R����ͬһ���ڣ�R��Y���γ����ֻ������Է�������֮��Ϊ4��5��Z��Wԭ�ӵĺ��������֮����Y��Rԭ�ӵĺ��������֮����ȣ����ƶϺ�ش��������⣺��1��Z��Y������ȼ�յIJ������ʽΪ
 �����к��еĻ�ѧ������Ϊ���Ӽ����ۼ���
�����к��еĻ�ѧ������Ϊ���Ӽ����ۼ�����2������Ԫ���γɵĵ������뾶�����ǣ�Na��
��3��W������Z�ĸ��������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
���� X�γɵĵ������ܶ���С�����ʣ�ӦΪ��������XΪHԪ�أ�Yԭ����������������������$\frac{3}{4}$����ӦΪOԪ�أ�Z��W��R����ͬһ���ڣ�R��Y���γ����ֻ������Է�������֮��Ϊ4��5��RΪSԪ�أ��γɵ����ֻ�����ΪSO2��SO3��Z��Wԭ�ӵĺ��������֮����Y��Rԭ�ӵĺ��������֮����ȣ�Ϊ24����ZΪNa��WΪAl����϶�Ӧ���ʵ������Լ�Ԫ�������ɵĵݱ���ɽ����⣮
��� �⣺��1�������Ϸ�����֪ZΪNa����������ȼ�����ɹ������ƣ�����ʽΪ ���������Ӽ����ۼ���
���������Ӽ����ۼ���
�ʴ�Ϊ�� �����Ӽ����ۼ���
�����Ӽ����ۼ���
��2��һ����˵��ԭ�Ӻ�����Ӳ���Խ�࣬ԭ�Ӱ뾶Խ��ͬ����Ԫ�غ˵����Խ��ԭ�Ӱ뾶ԽС��ԭ�Ӱ뾶����ΪNa���ʴ�Ϊ��Na��
��3��W����ΪAl����Z�ĸ��������Ӧ��ˮ����ΪNaOH������Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
���� ���⿼��λ�á��ṹ�����ʣ�Ϊ��Ƶ���㣬����ԭ�ӽṹ��Ԫ�ص�λ���ƶ�Ԫ��Ϊ���Ĺؼ���ע��Ԫ�������ɵ�Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
5������˵����ȷ���ǣ�������
| A�� | �ѻ�����������͵������͵IJ��������� | |
| B�� | Ӳ֬�������������Ӧ��õ�����Ҫ��Ʒ��Ӳ֬����� | |
| C�� | �Ҵ������ᡢ�������������о�����-OH | |
| D�� | ��2 mL���м���1 mL���CCl4��Һ�����ã��ϲ���Ϻ�ɫ |
10������������0.1mol�����ƣ����������һЩС�ף��������ˮ�г�ַ�Ӧ�������й�������ȷ���ǣ�������
| A�� | ��Ӧ������1.12L����״����H2 | |
| B�� | ��Ӧ�����Һ�м�������С�մ������а�ɫ�������� | |
| C�� | �ڷ�Ӧ�����Һ��һ���ܴ�������������У�K+��Mg2+��I-��NO3- | |
| D�� | ��Ӧ�����Һ�м�������ϡ���ᣬ�����а�ɫ�������� |
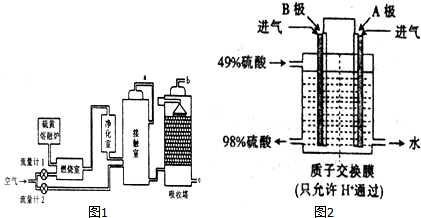
9�����������Ҫ����;��ij��������ͼ2ʾ�Ĺ��������������ᣮ
��ش��������⣺
��1������������Ϊԭ�ϵ�����������ȣ��ù��յ��ص���A��
A������������ B�����������ת�������
C�������ķ������� D������Ҫʹ�ô���
��2������a�ijɷ��Ƕ��������������
��3������Ӵ��ҵ����壬���Ⱦ��������ҵ�ԭ���Ƿ�ֹ��������еĻҳ���ˮ�������ڽӴ����е��´����ж���
��������������SO3������������98.3%Ũ���ᣮ
����SO2��O2Ϊԭ�ϣ����õ绯ѧԭ��Ҳ�����Ʊ����ᣬװ����ͼ2�缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���B���ĵ缫��ӦʽΪSO2-2e-+2H2O�TSO42-+4H+��
���������ų���β�����ð�ˮ���գ��õ����M������Ũ���� ����M���õ����{Ũ�ȵ�SO2��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2ʱ������Ӧ�����ӷ���ʽ��SO2-2e-+2H2O�TSO42-+4H+��
��Ϊȷ���������M�ijɷ֣�����ͬ��������ηֱ����50.00ml��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩����ñ�״����NH3����������
����εijɷ���NH4HSO3�ͣ�NH4��2SO3�������ʵ���֮����1��1��

��ش��������⣺
��1������������Ϊԭ�ϵ�����������ȣ��ù��յ��ص���A��
A������������ B�����������ת�������
C�������ķ������� D������Ҫʹ�ô���
��2������a�ijɷ��Ƕ��������������
��3������Ӵ��ҵ����壬���Ⱦ��������ҵ�ԭ���Ƿ�ֹ��������еĻҳ���ˮ�������ڽӴ����е��´����ж���
��������������SO3������������98.3%Ũ���ᣮ
����SO2��O2Ϊԭ�ϣ����õ绯ѧԭ��Ҳ�����Ʊ����ᣬװ����ͼ2�缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���B���ĵ缫��ӦʽΪSO2-2e-+2H2O�TSO42-+4H+��
���������ų���β�����ð�ˮ���գ��õ����M������Ũ���� ����M���õ����{Ũ�ȵ�SO2��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2ʱ������Ӧ�����ӷ���ʽ��SO2-2e-+2H2O�TSO42-+4H+��
��Ϊȷ���������M�ijɷ֣�����ͬ��������ηֱ����50.00ml��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩����ñ�״����NH3����������
| ������/g | 2.15 | 4.30 | 6.45 |
| NH3�����/mL | 672 | 1344 | 1344 |
16�����ſƼ��ķ��ٷ�չ���������������������ϣ���ֲ���������ڵ������մɲ���HAP[��ѧʽΪCam��PO4��n��OH��2]���ѱ�ҽ�����������˵Ĺ���������֯��HAP�Ļ�ѧʽ��m���ڣ�������
| A�� | $\frac{3n+2}{2}$ | B�� | $\frac{3n-2}{2}$ | C�� | $\frac{2n-2}{3}$ | D�� | n+1 |
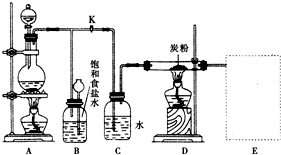 ��ͼ��һ��ʵ������ȡ������������Ϊԭ�Ͻ����ض���Ӧ��װ�ã�
��ͼ��һ��ʵ������ȡ������������Ϊԭ�Ͻ����ض���Ӧ��װ�ã�