��Ŀ����
3��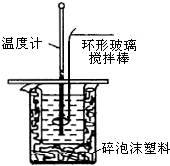 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25mol/L���ᵹ��С�ձ��У������¶ȣ�
����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȣ�
��ش�
��1��NaOH��Һ�Թ�����ԭ��ȷ�����ᱻ��ȫ�кͣ�
��2������NaOH��Һ����ȷ������B������ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�������û��β��������������
��4������Һ���ܶȾ�Ϊ1g/cm3���кͺ���Һ�ı�����c=4.18J/��g•�棩�������ʵ������д���÷�Ӧ���Ȼ�ѧ����ʽH2SO4��aq��+2NaOH��aq��Na2SO4��aq��+2H2O��l������H=-113.7kJ•mol-1��
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 25.0 | 25.2 | 25.1 | 28.5 | 3.4 |
| 2 | 24.9 | 25.1 | 25.0 | 28.3 | 3.3 |
| 3 | 25.6 | 25.4 | 25.5 | 29.0 | 3.5 |
���� ��1��Ϊ��ȷ�����������ᷴӦ��ȫ������NaOH�Թ�����
��2����NaOH��Һ����С�ձ��У����ּܷ��ε��룬����ᵼ������ɢʧ��Ӱ��ⶨ�����
��3��������������ƻ��ʱ���������¶ȼ��ϵĻ��β������������ؽ�����ʹ������NaOH��Һ��Ͼ��ȣ�
��4����������ε��²�ƽ��ֵ��Ȼ����ݹ�ʽQ=��H=��Tcm�����㷴Ӧ���ʱ䣬�������Ȼ�ѧ����ʽ����д������д�Ȼ�ѧ����ʽ��
��5������Ũ��������ˮ���������������
��� �⣺��1��ʵ���У�����NaOH�Թ�����ԭ����ȷ�����������ᷴӦ��ȫ��
�ʴ�Ϊ��ȷ�����ᱻ��ȫ�кͣ�
��2����������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ�����
�ʴ�Ϊ��B��
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ������¶ȼ��Dz����¶ȵģ�
�ʴ�Ϊ���û��β��������������
��4�����ε�ƽ���²�Ϊ��$\frac{3.4+3.3+3.5}{3}$��=3.4�棬Q=��H=-��Tcm=-3.4���4.18J/��g•�棩��100mL��1g/mL�T1.4212kJ/mol��50moL 0.25mol•L-1������50mL 0.55mol/L NaOH��Һ���кͷ�Ӧ����ˮ�����ʵ�����0.025mol����������2molˮ�ų�������Ϊ��$\frac{1.4212kJ}{0.025mol}$��2=113.7kJ•mol-1�������Ȼ�ѧ����ʽΪ��H2SO4��aq��+2NaOH��aq��Na2SO4��aq��+2H2O��l������H=-113.7kJ•mol-1��
�ʴ�Ϊ��H2SO4��aq��+2NaOH��aq��Na2SO4��aq��+2H2O��l������H=-113.7kJ•mol-1��
��5������Ũ��������ˮ�ų������������кͷ�Ӧ�з��ȵ�����ƫ��
�ʴ�Ϊ�����ڣ�Ũ��������ˮ�ų�������
���� ���⿼�����к��ȵIJⶨ��������Ŀ�Ѷ��еȣ�ע�����ղⶨ�к��ȵ���ȷ��������ȷʵ����������йؼ����ھ����ܼ�������ɢʧ��ʹ�ⶨ�������ȷ��

| Ԫ�� | �����Ϣ |
| X | ��X�γɵĵ�������������Դ |
| Y | Y�Ļ�̬ԭ�Ӻ���P�ܼ���������S�ܼ���һ�� |
| Z | ��Z�γɵĶ��ֵ��ʣ�����֮һ�ǵ���ġ�����ɡ�� |
| W | W�Ļ�̬ԭ�Ӻ��������ֻ��1�����ӣ��������������ʹ�õĻ��ҽ��� |
��2��Y�ĵ�һ�����ܱ�Z�Ĵ�����ߡ�С������YX3��X2Z�ķ����д��ڵĦҼ�֮��Ϊ3��2��Y�ļ��⻯����ˮ���о��˵��ܽ�ȣ�������ԭ��NH3��H2O����֮���γ������
��3��Y2X4��WZ�ڼ����������Ƶ�W���ʣ�д����ѧ����ʽ���������ת�Ƶķ�������Ŀ
 ��
�� 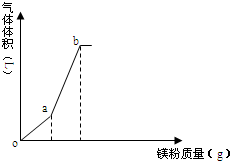 ��һ������NH4HSO4��Һ������þ�ۣ��������������þ�������仯��ͼ�������¶ȱ仯�������ܽ⣩�������й�˵����ȷ���ǣ�������
��һ������NH4HSO4��Һ������þ�ۣ��������������þ�������仯��ͼ�������¶ȱ仯�������ܽ⣩�������й�˵����ȷ���ǣ�������| A�� | o��ʱ����Һ�����Ե���Ҫԭ��Ϊ��NH4++H2O�TNH3•H2O+H+ | |
| B�� | oa����ҺpH����c��NH4+����С | |
| C�� | b����Һ������ | |
| D�� | a��b�����������Ϊ1��3 |
 ��������Ԫ�أ�����A��B��CΪ����������Ԫ�أ�D��EΪ��������Ԫ�أ����ǵ�ԭ������������������ݱ��������Ϣ���ش����⣮
��������Ԫ�أ�����A��B��CΪ����������Ԫ�أ�D��EΪ��������Ԫ�أ����ǵ�ԭ������������������ݱ��������Ϣ���ش����⣮| AԪ��ԭ�ӵĺ���p����������s����������1 |
| Bԭ�Ӻ�������p���ȫ������� |
| CԪ�ص������������������IJ�Ϊ4 |
| D��ǰ�������е縺����С��Ԫ�� |
| E�����ڱ��ĵ�ʮһ�� |
��2��E�����ڱ���λ���ǵ������ڵ�IB�壬λ��ds�������̬ԭ����29���˶�״̬��
��3��BC3����ԭ�ӵ��ӻ���ʽΪsp3���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ�����Σ�����DԪ�صķ�������ɫ��Ӧ��
��4��E���ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ12��E���ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е����������ѻ�������֪E��ԭ�Ӱ뾶Ϊd cm��NA���������ӵ�������E�����ԭ������ΪM����þ�����ܶ�Ϊ$\frac{M}{4\sqrt{2}{d}^{3}{N}_{A}}$g•cm-3������ĸ��ʾ����
| A�� | B��C���γ�һ�ֺ���ͬ���͵Ļ�ѧ���ļ��������� | |
| B�� | D2-������ˮ��Һ����Al3+��H+�����ܴ������� | |
| C�� | A2B���ȶ���ǿ��A2D���ȶ��� | |
| D�� | A2D��DB2�ɷ���������ԭ��Ӧ���������������뻹ԭ��������ʵ���֮��Ϊ2��1 |
| A�� | ���������ˮ��������ԣ��ң��� | |
| B�� | ���γɻ�������������Ԫ�� | |
| C�� | ��Ԫ��λ�ڵ�4���ڵ�VIIB�� | |
| D�� | �ҡ���������������ˮ������Է�Ӧ |
| A�� | X=0.6mol | |
| B�� | ���ӵ����ʵ�����ϵ����n��Cl-��=5n��ClO-��+n��ClO3-�� | |
| C�� | ����Ӧ��ת�Ƶĵ���Ϊn mol������0.3��n��0.5 | |
| D�� | ClO3-�����ʵ�����Χ�ǣ�0.1mol��n��ClO3-����0.3mol |
| A�� | 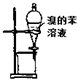 ��ͼװ����ȡ��ˮ�е��壬������ı���Һ���¿ڷų� | |
| B�� | 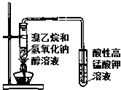 ��ͼװ�ÿ�����֤�����鷢������ȥ��Ӧ | |
| C�� |  ��ͼװ������������Һ | |
| D�� |  ��ͼװ�ÿ�˵��ŨH2SO4������ˮ�ԡ�ǿ�����ԣ�SO2����Ư���ԡ���ԭ�� |
| A�� | ��Ӧ��ϵ����ѹ�㶨 | B�� | A��B��Ũ����� | ||
| C�� | c��A����c��B��=1��3 | D�� | 2v��B����=3v��C���� |