题目内容
10.某同学取一定量的MgCl2固体溶于稀盐酸中,然后向此溶液中逐滴加入NaOH溶液,如图甲所示,滴加过程中产生沉淀的质量与NaOH溶液体积的关系如图乙所示.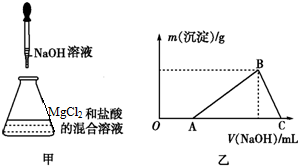
请回答下列问题:
(1)溶解MgCl2固体所用的玻璃仪器有bd(填字母).
a.天平 b.烧杯 c.漏斗 d.玻璃棒
(2)OA段反应的离子方程式为OH-+H+═H2O.
(3)AB段反应的离子方程式为3OH-+Al3+═Al(OH)3↓.
(4)在B点对应的溶液中滴加AgNO3溶液,观察到的现象是立即出现白色沉淀,反应的离子方程式为Cl-+Ag+═AgCl↓.
分析 (1)根据溶解操作方法判断使用的玻璃仪器;
(2)(3)(4)取一定量的MgCl2固体溶于稀盐酸中,然后向此溶液中逐滴加入NaOH溶液,首先发生反应OH-+H+═H2O,盐酸反应完毕,再发生反应反应3OH-+Al3+═Al(OH)3↓;最后发生NaOH+Al(OH)3=NaAlO2+2H2O,沉淀溶解,据此进行解答.
解答 解:首先发生NaOH+HCl═NaCl+H2O,盐酸反应完毕,再发生反应3NaOH+AlCl3=3NaCl+Al(OH)3↓,最后发生反应:OH-+Al(OH)3=AlO2-+2H2O,沉淀完全溶解,
(1)溶解AlCl3固体,应该在烧杯里溶解,并且用玻璃棒不断的搅拌,
故答案为:bd;
(2)根据以上分析OA段反应的离子方程式为:OH-+H+═H2O,
故答案:OH-+H+═H2O;
(3)根据以上分析AB段反应的离子方程式为:3OH-+Al3+═Al(OH)3↓,
故答案:3OH-+Al3+═Al(OH)3↓;
(4)根据以上分析B点时氢氧化铝刚好完全沉淀,溶液的只剩NaCl,所以滴加AgNO3溶液,发生反应为:Cl-+Ag+═AgCl↓,所以观察到的现象立即出现白色沉淀,
故答案:立即出现白色沉淀;Cl-+Ag+═AgCl↓.
点评 本题以图象形式考查离子反应的计算、混合物的有关计算,题目难度中等,明确各阶段发生的反应是解答关键,试题侧重考查学生的分析能力及灵活应用能力.

练习册系列答案
相关题目
5.在0.5L某浓度的NaCl溶液中含有0.5molNa+,下列对该溶液中的说法中,正确的是( )
①该溶液的物质的量浓度为1mol•L-1
②该溶液中含有58.5g NaCl
③配制100mL该溶液需用58.5g NaCl
④量取100mL该溶液倒入烧杯中,烧杯中Cl-的物质的量为0.1mol.
①该溶液的物质的量浓度为1mol•L-1
②该溶液中含有58.5g NaCl
③配制100mL该溶液需用58.5g NaCl
④量取100mL该溶液倒入烧杯中,烧杯中Cl-的物质的量为0.1mol.
| A. | ①② | B. | ②④ | C. | ②③④ | D. | ①④ |
15.下列说法中正确的是( )
| A. | CuSO4 •5H2O 是混合物 | |
| B. | 含Fe元素质量分数为70%的Fe2O3是纯净物 | |
| C. | 冰和水混合在一起形成混合物 | |
| D. | 由单质形成的物质一定是纯净物 |
4.向硝酸钡溶液逐渐通入二氧化硫气体,可能发生的离子方程式如下,其中错误的是( )
| A. | 3SO2+2NO3-+2H2O═2NO↑+4H++3SO42- | |
| B. | 3SO2+Ba2++2NO3-+2H2O═BaSO4↓+2NO↑+4H++SO42- | |
| C. | 6SO2+Ba2++4NO3-+4H2O═BaSO4↓+4NO↑+8H++5SO42- | |
| D. | 3SO2+3Ba2++2NO3-+2H2O═3BaSO4↓+2NO↑+4H+ |