��Ŀ����
3��ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�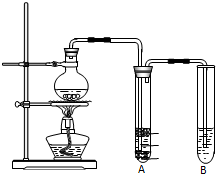
��1��Բ����ƿ�м���ķ�Ӧ�����廯�ơ��Ҵ���1��1�����ᣮ���������1��1���������õ�����Ϊb��d��ѡ���ţ���
a����ƽ b����Ͳ c������ƿ d���ձ�
��2������ʱ��ƿ�з�������Ҫ��Ӧ��������ѧ����ʽ��
��NaBr+H2SO4=HBr+NaHSO4
��HBr+CH3CH2OH $\stackrel{��}{��}$CH3CH2Br+H2O��
��3���������ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ��������������ȴ��Һ�������飮
��4����Ũ�������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ�������ʵ���ѷ�����d��ѡ���ţ���a������ b������������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ�ӣ�
���� ��1������������ʹ�÷�����ʵ���Ŀ����ѡ��������������ȡŨ��������Ͳ��ϡ�����ձ���
��2���������ʵ����ʽ�����д��Ӧ�Ļ�ѧ����ʽ���廯����Ҵ��ܷ���ȡ����Ӧ�����������ˮ��
��3��������Ϊ�����飬������ķе�Ϊ38.4��C��������������ˮ����ˮ���Խ��������������Һ̬�����飻
��4�����ݷ�Ӧ������ʼ�ѡ���и��Լ����ʽ��н��Ũ�������ǿ�����ԣ���������ԭ�������廯��Ϊ�嵥�ʵ����Թ�A�л�õ��л�����ػ�ɫ����ȥ�������е��壬���ܳ�ȥ�����飮
��� �⣺��1����ʵ����ȡ��Һ�����Ҫ��ȷ�Ȳ��Ǻܸߣ�������Ͳ��ȡ�����������1��1�����ᣬϡ��Ũ����ʱ��Ũ������ܶȱ�ˮ��Ҫ��Ũ���Ỻ����������ע��ˮ�У�ͬʱ�ò��������Ͻ��裬��ʹ������ʱ����ɢ�����Ի����ձ�����������
�ʴ�Ϊ��b��d��
��2��ҩƷ��Ϻ��ڼ�������������HBr�����Ҵ�����ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ��NaBr+H2SO4�THBr+NaHSO4��HBr+CH3CH2OH$\stackrel{��}{��}$CH3CH2Br+H2O��
�ʴ�Ϊ��HBr+CH3CH2OH $\stackrel{��}{��}$CH3CH2Br+H2O��
��3���ռ�װ�����õ����ܽϳ�����������ȴ�����ã����ɵ��������ˮ�������ܣ�������������ܶȱ�ˮ���ڱ�ˮ�������²㣬
�ʴ�Ϊ����ȴ��Һ�������飻
��4���Թ�A�л�õ��л�����ػ�ɫ��������Ũ�������ǿ�����ԣ���HBr������Br2�����Բ����������л��е����壬��������ȫ��ȥ���ʣ����Ҳ����鷳������������Һ��ʹ������ˮ�⣬���Ȼ�̼�������µ����ʣ����������ƺ��巢��������ԭ��Ӧ����HBr�������ƣ�������������룬����d��ȷ��
�ʴ�Ϊ��d��
���� ������Ҫ�����������Ʊ����ᴿ���л���Ӧ��֪ʶ������������ʵĻ�����ѧ���ʲ���Ϥ��װ�úͷ�Ӧԭ���ǽ����Ĺؼ���ƽʱ��ע�������ط�Ӧ֪ʶ�������Ѷ��еȣ�

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�| A�� | Na2C03 | B�� | HCl | C�� | Na2S04 | D�� | KN03 |
 ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�
ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/g•cm-3 | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֣�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g���ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ�Ũ��������ˮ��ų������ȣ����������ˣ�
��2�������ʯ�������Ƿ�ֹ���У�
��3������װ��ͼ�У�B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��4����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��5���¶ȼ�C1�����ò�����ƿ�з�Ӧ����¶ȣ�C2�����ò�����ֵķе㣮
 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}��Ũ��}$CH2=CH2
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�������������������Ҵ��Ʊ�1��2-���������װ�ü��й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
��1���ڴ��Ƹ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����B������ȷѡ��ǰ����ĸ��
A��������Ӧ B�����ٸ����� C����ֹ�Ҵ��ӷ�
��2����װ��C��Ӧ����C����Ŀ�������շ�Ӧ�п������ɵ��������壨����ȷѡ��ǰ����ĸ��
A������̼��������Һ B��Ũ���� C������������Һ
��3���ж�װ��D�и��Ʊ���Ӧ�Ѿ�������������ǹ۲���ˮ��ɫ�Ƿ��ʾ�
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ����¡���
��5�������������������������ѣ���ȥ���ѵķ�����������ʵ�����������
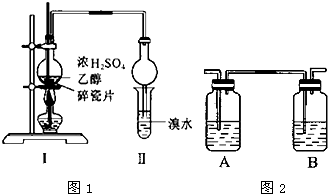 l��2��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.189cm-3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п������з�Ӧ�Ʊ�1��2�������飮
l��2��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.189cm-3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п������з�Ӧ�Ʊ�1��2�������飮 ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�