��Ŀ����
20��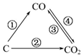 �������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�
�������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ���1����ͼΪC����������ı仯��ϵͼ�������û���Ӧ���ڢܾۢ�Ϊ���Ϸ�Ӧ����ٵĻ�ѧ����ʽ��ΪC+H2O=CO+H2��ͼ�б仯���������ȷ�Ӧ���Ǣ٢ۣ�����ţ���
��2���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO��g��+2H2��g��?CH3OH��g��
��������CO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��25�桢101kPa�£�1�˼״���ȫȼ������CO2��Һ̬ˮ������a kJ��д���״�ȼ���ȵ��Ȼ�ѧ����ʽ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
��3��������ұ������������һ����Ӧ�ǽ�ԭ�Ͻ��ʯת����TiO2�����ʯ��+2C+2Cl2=TiCl4+2CO����֪��
C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1��2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
TiO2��s��+2Cl2��g���TTiCl4��s��+O2��g����H=+141kJ•mol-1
��CO��ȼ������283 kJ/mol��
��1molCȼ�գ�$\frac{1}{2}$ת��ΪCO��$\frac{1}{2}$ת��ΪCO2����1molC���ȼ����ȣ���ʧ��������141.5kJ��
�۽����ʯת�����Ȼ�ѧ����ʽΪTiO2��s�����ʯ��+2C��s��+2Cl2��g��=TiCl4��s��+2CO��g����H=-80 kJ/mol��
���� ��1������̼���仯�������ʷ���ת����ϵ�������û���Ӧ������̼��ˮ������Ӧ����һ����̼��������������������ﷴӦ������̼��ˮ������Ӧ��̼�Ͷ�����̼��ӦΪ���ȷ�Ӧ��
��2������ȼ���ȸ�����1mol��ȼ����ȫȼ�������ȶ�������ų����������������������32g�״�ȼ�����ɶ�����̼��Һ̬ˮ���ȣ�����Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻
��3����ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų���������
����ʧ��������һ����̼ȼ�����ɶ�����̼�ų���������
�������Ȼ�ѧ����ʽ��˹���ɼ��������Ȼ�ѧ����ʽ��
��� �⣺��1�������û���Ӧ������̼��ˮ������Ӧ����һ����̼��������������������ﷴӦ����Ӧ�Ļ�ѧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2������̼��ˮ������Ӧ��̼�Ͷ�����̼��ӦΪ���ȷ�Ӧ���٢�Ϊ���ȷ�Ӧ��
�ʴ�Ϊ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2���٢ۣ�
��2����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����akJ��32g�״�ȼ�����ɶ�����̼��Һ̬ˮ�ų�����Ϊ32QKJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
��3����2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1��ȼ���ȵ��Ȼ�ѧ����ʽΪ��CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H=-283 kJ/mol��һ����̼ȼ����Ϊ283 kJ/mol��
�ʴ�Ϊ��283 kJ/mol��
��1molCȼ�գ�$\frac{1}{2}$ת��ΪCO��$\frac{1}{2}$ת��ΪCO2����1molC���ȼ����ȣ���ʧ��������һ����̼ȼ�����ɶ�����̼�ų�������������Ȼ�ѧ����ʽ�Ͷ�����ϵ����õ���C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1��CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H=-283kJ•mol-1
$\frac{1}{2}$molCת��ΪCOȼ�շ���$\frac{283KJ}{2}$=141.5kJ/mol��
�ʴ�Ϊ��141.5��
�ۢ�C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1��
��2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
��TiO2��s��+2Cl2��g���TTiCl4��s��+O2��g����H=+141kJ•mol-1
��˹���ɼ�����2-��+��õ���TiO2��s�����ʯ��+2C��s��+2Cl2��g��=TiCl4��s��+2CO��g����H=-80 kJ/mol��
�ʴ�Ϊ��TiO2��s�����ʯ��+2C��s��+2Cl2��g��=TiCl4��s��+2CO��g����H=-80 kJ/mol��
���� ���⿼�����Ȼ�ѧ����ʽд����˹���ɼ��������ȼ���ȸ�������Ӧ�ã����ռ��㷽�����Ȼ�ѧ����ʽ����д�����ǹؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�
| A�� | ����BaCl2��Һ����ϡ���� | B�� | ����ϡ���ᣬ��ϡBaCl2��Һ | ||
| C�� | ����ϡ���ᣬ����AgNO3��Һ | D�� | ����ϡ���ᣬ����AgNO3��Һ |
| �缫��Ӧʽ | ���ֻ��� | |
| A | O2+2H2O+4e-�T4OH- | ���Ի���������ȼ�ϵ�صĸ�����Ӧ |
| B | 4OH--4e-�T2H2O+O2�� | �����Ի����¸�����������ʴ |
| C | 2H++2e-�TH2�� | �ö��Ե缫���NaOH��Һ��������Ӧ |
| D | H2-2e-�T2H+ | �ö��Ե缫���H2SO4��������Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | H2O | B�� | NaCl | C�� | SO2 | D�� | HCl |
| A�� |  �������� | B�� |  ����K2CO3�е�K+ | ||
| C�� |  �ռ�������NH3������ | D�� |  �Ʊ�Fe��OH��2 |
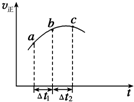 ����Ⱥ����ܱ�������ͨ��SO2��NO2һ��������ʹ��ӦSO2��g��+NO2��g��?SO3��g��+NO��g���ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ����ͼ�ɵó�����ȷ�����ǣ�������
����Ⱥ����ܱ�������ͨ��SO2��NO2һ��������ʹ��ӦSO2��g��+NO2��g��?SO3��g��+NO��g���ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ����ͼ�ɵó�����ȷ�����ǣ�������| A�� | ��t1=��t2ʱ��SO2��ת���ʣ�a��b��С��b��c�� | |
| B�� | ��Ӧ�������������������������� | |
| C�� | ��Ӧ��Ũ�ȣ�a��С��b�� | |
| D�� | ��Ӧ��C��ﵽƽ��״̬ |
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | M������2�ԳɶԵ��� |
| X | �����������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | Ԫ�����������+7�� |
��2��Ԫ��Y����Ԫ���γ�һ������YH${\;}_{4}^{+}$��д�������ĵ���ʽ
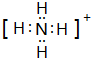 ����Ԫ�ط��ű�ʾ����
����Ԫ�ط��ű�ʾ������3��Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ����Cl����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����bef��
a��������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b��Z���⻯���T���⻯���ȶ�
c��һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d��Zԭ�ӵļ۵�������Tԭ�Ӷ�
e��Z������������ˮ��������ǿ��T������������ˮ���
f��Z�ĵ�������T���⻯��ˮ��Һ�����û���Ӧ
��4��Z�ж��ֳ��������ᣬ������ǿ������˳����HClO4��HClO3��HClO��
| A�� | Һ̬�������д����������������ӱ��Ȼ�����ȶ� | |
| B�� | ��̼����ԭ���γɵĻ�����Ƚ��ʯӲ������Ҫԭ����̼������̼̼������ | |
| C�� | S8��NO2���ǹ��ۻ����NH4Cl��CaC2�������ӻ����� | |
| D�� | ����AijԪ�ص�ԭ������Ϊm����ͬ���ڢ�AԪ�ص�ԭ�������п���Ϊm+10 |
| A�� | x-3 | B�� | x+3 | C�� | x+11 | D�� | x-11 |