��Ŀ����
����Ŀ��������Ҫ�ĺϽ���Ϻʹ������ڹ�ũҵ�����ͿƼ������й㷺����;����ش��������⣺
��1����Һ�е�Mn2+�ɱ�����![]() ��Һ����ΪMnO4-���÷��������ڼ���Mn2+��
��Һ����ΪMnO4-���÷��������ڼ���Mn2+��
�ټ���ʱ��ʵ������Ϊ_________��
�ڸ÷�Ӧ�����ӷ���ʽΪ___________��
�� ![]() �ɿ�������������ż�����ã�������ĽṹʽΪ
�ɿ�������������ż�����ã�������ĽṹʽΪ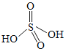 ����
����![]() �ĽṹʽΪ_________��
�ĽṹʽΪ_________��
��2��ʵ�����ú��̷��ϣ���Ҫ�ɷ�![]() ����������
����������![]() ���Ʊ�Mn���������£�
���Ʊ�Mn���������£�
��֪������������ܶȻ��������±���ʾ��
������ |
|
|
|
|
�ܶȻ������� | 4.0��10��38 | 1.0��10��33 | 1.8��10��11 | 1.8��10��13 |
����Һ������Ũ����10��5 mol��L��1ʱ����Ϊ�����ӳ�����ȫ��
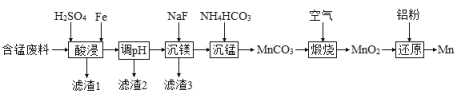
���������ʱ��![]() ��Fe����ΪFe3�����÷�Ӧ�����ӷ���ʽΪ________���ù�����ʱ���Һ�̱ȶ��̽����ʵ�Ӱ��ֱ���ͼ1��ͼ2��ʾ��
��Fe����ΪFe3�����÷�Ӧ�����ӷ���ʽΪ________���ù�����ʱ���Һ�̱ȶ��̽����ʵ�Ӱ��ֱ���ͼ1��ͼ2��ʾ��
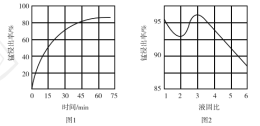
�����˵Ľ���ʱ���Һ�̱ȷֱ�Ϊ___________��___________��
�������������������Һ��c(Mn2��)��0.18 mol��L��1������pH���ķ�ΧΪ___________��
������������Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_______������ԭ��ʱ�������ķ�Ӧ�ڻ�ѧ���ֽ���_________��
���𰸡���Һ����ɫ��Ϊ�Ϻ�ɫ 5S2O82-+2Mn2++8H2O=2MnO4-+10SO42-+16H+ ![]() 3MnO2+2Fe+12H+=3Mn2++2Fe3��+6H2O 60min 3��1
3MnO2+2Fe+12H+=3Mn2++2Fe3��+6H2O 60min 3��1 ![]()
![]() pH��8 1��2 ���ȷ�Ӧ
pH��8 1��2 ���ȷ�Ӧ
��������
��1������Һ�е�Mn2+ת��ΪMnO4-��ʵ������Ϊ��Һ����ɫ��Ϊ�Ϻ�ɫ���ʴ�Ϊ����Һ����ɫ��Ϊ�Ϻ�ɫ��
��Mn2+������(NH4)2S2O8��Һ������MnO4-����ԭ����ӦΪSO42-����Ӧ�����ӷ���ʽΪ��5S2O82-+2Mn2++8H2O=2MnO4-+10SO42-+16H+���ʴ�Ϊ��5S2O82-+2Mn2++8H2O=2MnO4-+10SO42-+16H+��
��H2S2O8����Ϊ�������ӵ������������ã�����Ľṹ��ʽΪ��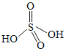 ����H2S2O8�Ľṹ��ʽΪ��
����H2S2O8�Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2������������Ϣ��֪���������ʱ��MnO2�����Խ����н�Fe����ΪFe3������������ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ��3MnO2+2Fe+12H+=3Mn2++2Fe3��+6H2O����ͼ��֪�����˵Ľ���ʱ��Ϊ60min����ͼ�ҿ�֪�����˵�Һ�̱�Ϊ3��1���ʴ�Ϊ��3MnO2+2Fe+12H+=3Mn2++2Fe3��+6H2O��60min��3��1��
��������ͼ����Ϣ��֪������pH����Ŀ����ʹFe3����Al3��������ȫ����Mn2��������������Ksp[Al(OH)3]=1.0��10��33��Ksp[Fe(OH)3]=4.0��10��38��֪��Al3��������ȫʱFe3���ѳ�����ȫ��Al(OH)3ǡ����ȫ����ʱ��pH=-lg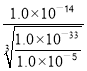 =
=![]() ��Mn2����ʼ����ʱ��pHΪ-lg
��Mn2����ʼ����ʱ��pHΪ-lg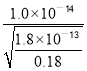 =8����pH���ķ�ΧΪ
=8����pH���ķ�ΧΪ![]()
![]() pH��8���ʴ�Ϊ��
pH��8���ʴ�Ϊ��![]()
![]() pH��8��
pH��8��
�۸�����Ϣ��֪�������ա�ʱ�������е�O2��MnCO3����ΪMnO2�����ݵ�ʧ�����غ�ɵù�ϵʽO2��2MnCO3�����������ͻ�ԭ�������ʵ���֮��Ϊ1��2������ԭ��ʱ������������۵����������ķ�Ӧ�ڻ�ѧ���ֽ������ȷ�Ӧ���ʴ�Ϊ1��2�����ȷ�Ӧ��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�