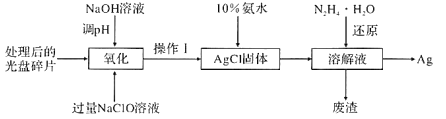
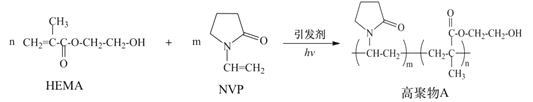
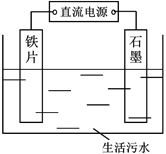
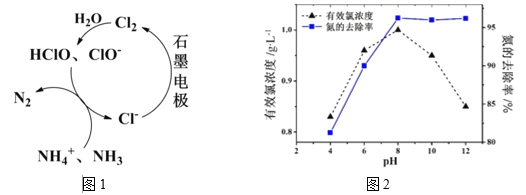
ΧβΡΩΡΎ»ί
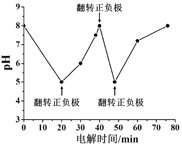
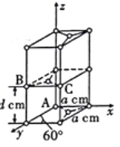
ΓΨΧβΡΩΓΩ“―÷ΣAΓΔBΓΔCΓΔDΚΆEΕΦ «‘ΣΥΊ÷ήΤΎ±μ÷–«Α36Κ≈ΒΡ‘ΣΥΊΘ§ΥϋΟ«ΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΓΘA”κΤδΥϊ4÷÷‘ΣΥΊΦ»≤Μ‘ΎΆ§“Μ÷ήΤΎ”÷≤Μ‘ΎΆ§“Μ÷ςΉεΓΘBΚΆC τΆ§“Μ÷ςΉεΘ§DΚΆE τΆ§“Μ÷ήΤΎΘ§”÷÷ΣE «÷ήΤΎ±μ÷–1ΓΪ18Ν–÷–ΒΡΒΎ7Ν–‘ΣΥΊΓΘDΒΡ‘≠Ή”–ρ ΐ±»E–Γ5Θ§DΗζBΩ…–Έ≥…άκΉ”Μ·ΚœΈοΘ§ΤδΨßΑϊΫαΙΙ»γΆΦΓΘ
«κΜΊ¥πΘΚ
Θ®1Θ©A‘ΣΥΊΒΡΟϊ≥Τ «______ΓΘ
Θ®2Θ©BΒΡ‘ΣΥΊΖϊΚ≈ «__________Θ§CΒΡ‘ΣΥΊΖϊΚ≈ «______ΓΘ
Θ®3Θ©E τ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎ________÷ήΤΎΒΎ______ΉεΒΡ‘ΣΥΊΘ§Τδ‘ΣΥΊΟϊ≥Τ «______Θ§ΥϋΒΡΘΪ2ΦέάκΉ”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ________ΓΘ
Θ®4Θ©¥”ΆΦ÷–Ω…“‘Ω¥≥ωΘ§DΗζB–Έ≥…ΒΡάκΉ”Μ·ΚœΈοΒΡΜ·―ß ΫΈΣ______ΘΜΗΟάκΉ”Μ·ΚœΈοΨßΧεΒΡΟήΕ»ΈΣa gΓΛcmΘ≠3Θ§‘ρΨßΑϊΒΡΧεΜΐ «______(÷Μ“Σ«σΝ–≥ωΥψ Ϋ)ΓΘ
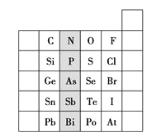
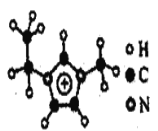
ΓΨ¥πΑΗΓΩ«β F Cl 4 ΔςB ΟΧ‘ΣΥΊ 1s22s22p63s23p63d5(Μρ[Ar]3d5) CaF2 ![]()
ΓΨΫβΈωΓΩ
¥”DΓΔE «÷ήΤΎ±μ÷–1-18Ν–÷–E≈≈ΒΎ7Ν–Ω…≈–ΕœE «ΒΎ4÷ήΤΎVIIBΉεΒΡMn‘ΣΥΊΘ§‘≠Ή”–ρ ΐΈΣ25Θ§Υυ“‘D“≤‘ΎΒΎ4÷ήΤΎΘ§DΒΡ‘≠Ή”–ρ ΐ±»E–Γ5Θ§‘ρ‘≠Ή”–ρ ΐΈΣ20Θ§”ΠΈΣCa‘ΣΥΊΘ§ΆΦ÷–άκΉ”Μ·ΚœΈοD”κBΒΡάκΉ”Ηω ΐ±»÷ΒΈΣΘΚΘ®8ΓΝ1/8+6ΓΝ1/2Θ©ΘΚ8=1ΘΚ2Θ§«“DΈΣCaΘ§‘ρBΒΡΜ·ΚœΦέΈΣ-1ΦέΘ§”ΠΈΣΒΎΔςAΉε‘ΣΥΊΘ§BΚΆC τΆ§“Μ÷ςΉεΘ§BΒΡ–ρ ΐ‘Ύ«ΑΟφΘ§BΈΣFΘ§CΈΣClΘΜA”κΤδΥϊ4÷÷Θ®1Θ©‘ΣΥΊΦ»≤Μ‘ΎΆ§“Μ÷ήΤΎ”÷≤Μ‘ΎΆ§“Μ÷ςΉεΘ§Υυ“‘AΈΣHΘΜ
Θ®1Θ©AΈΣH‘ΣΥΊΘ§Οϊ≥ΤΈΣ«βΓΘ
Θ®2Θ©BΒΡ‘ΣΥΊΖϊΚ≈ «F Θ§CΒΡ‘ΣΥΊΖϊΚ≈ «ClΘΜ
Θ®3Θ©ΗυΨί‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ≈–Εœ‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷ΟΘ§ΗυΨίΡήΝΩΉνΒΆ‘≠άμΚΆΚιΧΊΙφ‘ρ ι–¥άκΉ”ΒΡΒγΉ”≈≈≤Φ ΫΘΜ
Θ®4Θ©άϊ”ΟΨυΧ·Ζ®ΦΤΥψΜ·―ß ΫΘΜΗυΨίΠ―=m/VΦΤΥψΨßΑϊΒΡΧεΜΐΘ°ΘΜ
Θ®1Θ©AΈΣH‘ΣΥΊΘ§Οϊ≥ΤΈΣ«β;
Θ®2Θ©BΒΡ‘ΣΥΊΖϊΚ≈ «F Θ§CΒΡ‘ΣΥΊΖϊΚ≈ «ClΘΜ
Θ®3Θ©EΈΣMn‘ΣΥΊΘ§ΈΜ”Ύ÷ήΤΎ±μΒΎΥΡ÷ήΤΎΒΎΤΏΝ–Θ§‘ρ”ΠΈΜ”ΎΔςBΉεΘ§‘≠Ή”ΒΡΚΥΆβΒγΉ”ΒΡ≈≈≤Φ ΫΈΣ1s22s22p63s23p63d54s2(Μρ[Ar]3d54s2) Θ§ ß»Ξ2ΗωΒγΉ”Θ§άκΉ”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d5(Μρ[Ar]3d5) .
Θ®4Θ©¥”ΆΦ÷–Ω…“‘Ω¥≥ωΘ§ΨßΑϊ÷–Κ§”–CaΒΡάκΉ”Ηω ΐΈΣ8ΓΝ1/8+6ΓΝ1/2=4Θ§Κ§”–FΒΡάκΉ”Ηω ΐΈΣ8Θ§Εΰ’Ώ±»÷ΒΈΣ1ΘΚ2Θ§‘ρΜ·―ß ΫΈΣCaF2ΘΜΨßΑϊ÷–Θ§Π―=m/V=![]() =agΓΛcm-3Θ§‘ρV=
=agΓΛcm-3Θ§‘ρV=![]()

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΟΧ «÷Ί“ΣΒΡΚœΫπ≤ΡΝœΚΆ¥ΏΜ·ΦΝΘ§‘ΎΙΛ≈©“Β…ζ≤ζΚΆΩΤΦΦΝλ”ρ”–ΙψΖΚΒΡ”ΟΆΨΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©»ή“Κ÷–ΒΡMn2+Ω…±ΜΥα–‘![]() »ή“Κ―θΜ·ΈΣMnO4-Θ§ΗΟΖΫΖ®Ω…”Ο”ΎΦλ―ιMn2+ΓΘ
»ή“Κ―θΜ·ΈΣMnO4-Θ§ΗΟΖΫΖ®Ω…”Ο”ΎΦλ―ιMn2+ΓΘ
ΔΌΦλ―ι ±ΒΡ Β―ιœ÷œσΈΣ_________ΓΘ
ΔΎΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___________ΓΘ
Δέ ![]() Ω…Ω¥≥…ΝΫΖ÷Ή”ΝρΥα≈ΦΚœΥυΒΟΘ§»τΝρΥαΒΡΫαΙΙ ΫΈΣ
Ω…Ω¥≥…ΝΫΖ÷Ή”ΝρΥα≈ΦΚœΥυΒΟΘ§»τΝρΥαΒΡΫαΙΙ ΫΈΣ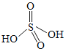 Θ§‘ρ
Θ§‘ρ![]() ΒΡΫαΙΙ ΫΈΣ_________ΓΘ
ΒΡΫαΙΙ ΫΈΣ_________ΓΘ
Θ®2Θ© Β―ι “”ΟΚ§ΟΧΖœΝœΘ®÷ς“Σ≥…Ζ÷![]() Θ§Κ§”–…ΌΝΩ
Θ§Κ§”–…ΌΝΩ![]() Θ©÷Τ±ΗMnΒΡΝς≥Χ»γœ¬ΘΚ
Θ©÷Τ±ΗMnΒΡΝς≥Χ»γœ¬ΘΚ
“―÷ΣΘΚΔώΘ°Ρ―»ήΈοΒΡ»ήΕ»Μΐ≥Θ ΐ»γœ¬±μΥυ ΨΘΚ
Ρ―»ήΈο |
|
|
|
|
»ήΕ»Μΐ≥Θ ΐΘ® | 4.0ΓΝ10Θ≠38 | 1.0ΓΝ10Θ≠33 | 1.8ΓΝ10Θ≠11 | 1.8ΓΝ10Θ≠13 |
ΔρΘ°»ή“Κ÷–άκΉ”≈®Ε»Γή10Θ≠5 molΓΛLΘ≠1 ±Θ§»œΈΣΗΟάκΉ”≥ΝΒμΆξ»ΪΓΘ
ΔΌΓΑΥαΫΰΓ± ±Θ§![]() ΫΪFe―θΜ·ΈΣFe3ΘΪΓΘΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________ΘΜΗΟΙΐ≥Χ÷– ±ΦδΚΆ“ΚΙΧ±»Ε‘ΟΧΫΰ≥ω¬ ΒΡ”ΑœλΖ÷±π»γΆΦ1ΓΔΆΦ2Υυ ΨΘΚ
ΫΪFe―θΜ·ΈΣFe3ΘΪΓΘΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________ΘΜΗΟΙΐ≥Χ÷– ±ΦδΚΆ“ΚΙΧ±»Ε‘ΟΧΫΰ≥ω¬ ΒΡ”ΑœλΖ÷±π»γΆΦ1ΓΔΆΦ2Υυ ΨΘΚ
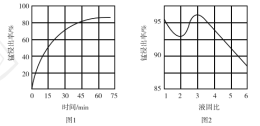
‘ρ “ΥΒΡΫΰ≥ω ±ΦδΚΆ“ΚΙΧ±»Ζ÷±πΈΣ___________ΓΔ___________ΓΘ
ΔΎ»τΓΑΥαΫΰΓ±ΚσΥυΒΟ¬Υ“Κ÷–c(Mn2ΘΪ)ΘΫ0.18 molΓΛLΘ≠1Θ§‘ρΒςpHΓ±ΒΡΖΕΈßΈΣ___________ΓΘ
ΔέΓΑλ―…’Γ±Ζ¥”Π÷–―θΜ·ΦΝΚΆΜΙ‘≠ΦΝΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ_______ΓΘΓΑΜΙ‘≠Γ± ±ΥυΖΔ…ζΒΡΖ¥”Π‘ΎΜ·―ß…œ”÷Ϋ–Ήω_________ΓΘ