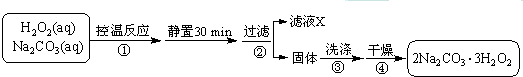
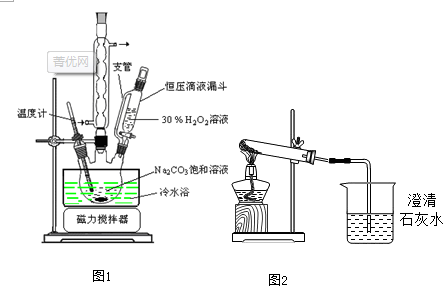
��Ŀ����
8��ijС��������ͼװ�ã��ñ�������FeBr3���������Ʊ��屽��
��Ӧ���ҽ��У���ƿ���д�������ɫ��������ƿ�е��ܿ��а������֣�����ˮ��ɻ�ɫ����Ӧֹͣ�� �������̷����Ʒ��

��֪��
| �е�� | �ܶ�g/cm3 | �ܽ��� | |
| �� | 59 | 3.119 | ˮ���ܽ��С���������л��ܼ� |
| �� | 80 | 0.8765 | ������ˮ�����л��ܼ����� |
| �屽 | 156 | 1.50 | ������ˮ�����л��ܼ����� |
��2����ˮϴ������NaOH��Һϴ����Ҫ�õ��IJ��������Ƿ�Һ©�����ձ���
��3����ˮϴ����ҪĿ���dz�ȥFeBr3����NaOH��Һϴ����ҪĿ���dz�ȥBr2��
��4����ƿ������ˮ��Ƶ�ԭ�����ܽ��˴���ƿ�лӷ������壮
��5����֪�����巢������ȡ����Ӧ���ƲⷴӦ����ƿ��Һ�庬�е����ִ���������Br-��H+�������ʵ�鷽����֤����Ʋ⣮����ѡ�Լ���þ�������Ȼ�̼����ˮ����ˮ������ˮ��
| ��� | ʵ�鲽�� | Ԥ������ | ���� |
| 1 | ����ƿ��Һ��ת���Һ©���������������Ȼ�̼�����Һ���ֱ�ȡ�����ϲ���ɫ��Һ���Թ�A��B�� | ||
| 2 | ��ƿ��Һ�庬���� Br- | ||
| 3 | ��ƿ��Һ�庬���� H+ |
���� ��1����2����3����������FeBr3���������Ʊ��屽����Ӧ������к��б����屽���塢FeBr3�Լ�Fe�������̿�֪���������õ����������������ӦΪ���ˣ����������ΪFe�ۣ�Һ���к���FeBr3�ȣ�������ˮ��ˮϴ����з�Һ���룬�л����к��б����屽���壬��������������Һ��ȥBr2���پ�����Һ���룬ˮ������Ҫ����NaBr��NaBrO�ȣ��л����к��б����屽�����ڶ��߷е㲻ͬ���ɽ���������룻
��4�����ӷ����ӷ�����������ƿ��ˮ�У�ʹ����ˮ��ƣ�
��5�������巢������ȡ����Ӧ����Ӧ����HBr������ƿ�У���Һ�к��д�����Br-��H+���������ӷ�����ƿ�л��ܽ��������壬�����Ȼ�̼��Һ��ȡ���룬������ˮ�û����嵥�ʼ���Br-���ӣ�����Mg���ᷴӦ�����������H+���ӣ�
��� �⣺��������FeBr3���������Ʊ��屽����Ӧ������к��б����屽���塢FeBr3�Լ�Fe�������̿�֪���������õ����������������ӦΪ���ˣ����������ΪFe�ۣ�Һ���к���FeBr3�ȣ�������ˮ��ˮϴ����з�Һ���룬�л����к��б����屽���壬��������������Һ��ȥBr2���پ�����Һ���룬ˮ������Ҫ����NaBr��NaBrO�ȣ��л����к��б����屽�����ڶ��߷е㲻ͬ���ɽ���������룮
��1�������Ϸ�����֪������Ϊ���ˣ�������Ϊ���ʴ�Ϊ�����ˣ�����
��2������ˮϴ������NaOH ��Һϴ����õ�ˮ����л��࣬��ӦΪ��Һ����������Ҫ�ձ��⣬����Ҫ��Һ©�����ʴ�Ϊ����Һ©����
��3����ˮϴ����ҪĿ���dz�ȥ FeBr3����NaOH��Һϴ����ҪĿ���dz�ȥBr2���ʴ�Ϊ��FeBr3��Br2��
��4���������ӷ�����ƿ��ˮ�ܽ��˴���ƿ�лӷ����������ƣ��ʴ�Ϊ���ܽ��˴���ƿ�лӷ������壻
��5�������巢������ȡ����Ӧ����Ӧ����HBr������ƿ�У���Һ�к��д�����Br-��H+���������ӷ�����ƿ�л��ܽ��������壬�����Ȼ�̼��Һ��ȡ���룬�ֱ�ȡ�����ϲ���ɫ��Һ���Թ�A��B�У��Թ�A�е���������ˮ���û����嵥�ʣ���Һ����ɫ���ɫ��֤������Br-���ӣ���B�Թ��м���Mg�����д����������ɣ�֤������H+���ӣ�
�ʴ�Ϊ��Br-��H+��
| 2 | ���Թ�A�м���������ˮ | ��Һ����ɫ���ɫ | Br- |
| 3 | ���Թ�B�м���þ�� | �д����������� | H+ |
���� ���⿼���л�����Ʊ��Լ����ʵķ��롢�ᴿ��������ѧ���ķ�����ʵ�������Ŀ��飬�ؼ�������ʵ���ԭ���Լ�ʵ�����̣��Ѷ��еȣ�

 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�| A�� | ��ѧƽ�ⳣ���Ĵ�С���¶ȡ�Ũ�ȡ�ѹǿ�йأ�������� | |
| B�� | ��֪��K1 ��H2CO3����Ka ��HClO����K2��H2CO3������NaClO��Һ��ͨ������CO2�Ļ�ѧ�����ǣ�2NaClO+CO2+H2O=Na2CO3+2HClO | |
| C�� | 25��ʱ��pH=4�������У�KW=10-20 | |
| D�� | �����£�Ksp��CaSO4��=9��10-6����100mL����CaSO4��Һ�м�400mL 0.01mol/LNa2SO4��Һ���������� |
| A�� | NaCl | B�� | C | C�� | Fe | D�� | Ar |
| A�� | ��ɡ����ꡱ��������� | B�� | ˮ��Һ�ʼ��� | ||
| C�� | ������Ʒ����Һ��ɫ | D�� | ����ʹ�����ʯ��ˮ����� |
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
 ��
����2���������γ��������������Ԫ����������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������Ϊ���û�ѧʽ��ʾ��KOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
��4����Ԫ�����Ԫ�����ߺ˵����֮����26��
��5����д���ڵ��⻯����������Ļ�ѧ����ʽ4NH3+5O2
 4NO+6H2O��
4NO+6H2O�� 
| A�� | �������һ��������FeCl2��NaCl | |
| B�� | ��Ӧ�ܵ����ӷ���ʽΪ��AlO2-+H++H2O=Al��OH��3�� | |
| C�� | �������һ������Al����NH4��2SO4��MgCl2�������ʣ����ж��Ƿ���AlCl3 | |
| D�� | ��ɫ����5.80g��Mg��OH��2 |
����˵���д������ ��������
| A�� | PFS������+3�� | |||||||||||||||
| B�� | ��ԭ�ӵļ۵����Ų�ʽ��3d74s1 | |||||||||||||||
| C�� | ��FeSO4��Һ��PFS�辭��������ˮ��;ۺϵĹ��� | |||||||||||||||
| D�� | ���±���֪��̬Fe2+��ʧȥһ�����ӱ���̬Mn2+��ʧȥһ��������
| |||||||||||||||
| A�� | SO42- | B�� | NH4+ | C�� | H+ | D�� | OH- |