��Ŀ����
16��K��Ka��Kb��KW��Ksp�ֱ��ʾ��ѧƽ�ⳣ�������볣����ˮ�����ӻ����ܶȻ����������й�����Щ������˵���У���ȷ���ǣ�������| A�� | ��ѧƽ�ⳣ���Ĵ�С���¶ȡ�Ũ�ȡ�ѹǿ�йأ�������� | |
| B�� | ��֪��K1 ��H2CO3����Ka ��HClO����K2��H2CO3������NaClO��Һ��ͨ������CO2�Ļ�ѧ�����ǣ�2NaClO+CO2+H2O=Na2CO3+2HClO | |
| C�� | 25��ʱ��pH=4�������У�KW=10-20 | |
| D�� | �����£�Ksp��CaSO4��=9��10-6����100mL����CaSO4��Һ�м�400mL 0.01mol/LNa2SO4��Һ���������� |
���� A����ѧƽ�ⳣ��ֻ���¶��йأ�
B����֪K1��H2CO3����Ka��HClO����K2��H2CO3������������H2CO3��HClO��HCO3-���ʲ���ֻ����̼�����ƣ�
C��ˮ�����ӻ�ֻ���¶��йأ�25��ʱ��KW=10-14��
D��CaSO4������Һc��Ca2+��=c��SO42-��=3��10-3mol/L������400mL 0.01mol/LNa2SO4��Һ��c��Ca2+��=0.6��10-3mol/L��c��SO42-��=8.6��10-3mol/L���Ƚ�Qc��Ksp��
��� �⣺A����ѧƽ�ⳣ��ֻ���¶��йأ���Ũ�ȡ�ѹǿ�������أ���A����
B������H2CO3��HClO��HCO3-������CO2ͨ�����������Һ�з�Ӧ����̼�����ƺʹ����ᣬ��ѧ����ʽΪ��CO2+H2O+NaClO�TNaHCO3+HClO����B����
C��ˮ�����ӻ�ֻ���¶��йأ�25��ʱ��KW=10-14��pH=4�������У�KW��ȻΪ10-14����C����
D��CaSO4������Һc��Ca2+��=c��SO42-��=3��10-3mol/L������400mL 0.01mol/LNa2SO4��Һ���Һ��c��Ca2+��=$\frac{0.1L��3.0��1{0}^{-3}mol/L}{0.1L+0.4L}$=6.0��10-4 mol/L��c��SO42-��=$\frac{0.1L��3.0��1{0}^{-3}mol/L+0.4L��0.01mol/L}{0.1L+0.4L}$=8.6��10-3 mol/L��
��Һ��c��Ca2+��•c�� SO42- ��=5.16��10-6��Ksp��CaSO4��=9.0��10-6�����Ի��Һ��������������D��ȷ��
��ѡ��D��
���� ���⿼�黯ѧƽ�ⳣ�������볣�����ܶȻ��ȣ��Ѷ��еȣ�ע�����ո��ֳ�������ʽ����д��Ӱ�����أ�

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�| Z | |
| X | Y |
| A�� | �����ǵ�ԭ������������֮��Ϊ11����XΪ����Ԫ�� | |
| B�� | ����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳��һ����Y��X��Z | |
| C�� | �����Ǿ�Ϊ����Ԫ�أ���Y������������Ӧˮ����ļ�����ǿ | |
| D�� | ZԪ�ص�����ϼ�һ������XԪ�� |
| A�� | HCO3-�����Զ���CO32-���� | |
| B�� | HCO3-���ӵ���CO32-���� | |
| C�� | HCO3-����������CO32-���� | |
| D�� | Na+����������HCO3-��CO32-������֮�� |
| A�� | C2O3��CO2��ͬ�������� | B�� | C2O3��CO��ȫȼ�գ����ﶼ��CO2 | ||
| C�� | C2O3��CO �������������� | D�� | C2O3��CO2����̼������� |
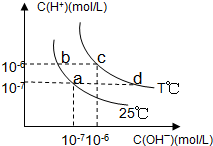 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������| A�� | a���Ӧ����Һ�д������ڣ�Fe3+��Na+��SO42-��Cl- | |
| B�� | b���Ӧ����Һ�д������ڣ�Fe2+��Cl-��NO3-��Na+ | |
| C�� | c���Ӧ����Һ�д������ڣ�Ba2+��Cl-��Na+��Br- | |
| D�� | d���Ӧ����Һ�д������ڣ�Cu2+��K+��SO42-��NO3- |
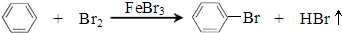
��Ӧ���ҽ��У���ƿ���д�������ɫ��������ƿ�е��ܿ��а������֣�����ˮ��ɻ�ɫ����Ӧֹͣ�� �������̷����Ʒ��
��֪��
| �е�� | �ܶ�g/cm3 | �ܽ��� | |
| �� | 59 | 3.119 | ˮ���ܽ��С���������л��ܼ� |
| �� | 80 | 0.8765 | ������ˮ�����л��ܼ����� |
| �屽 | 156 | 1.50 | ������ˮ�����л��ܼ����� |
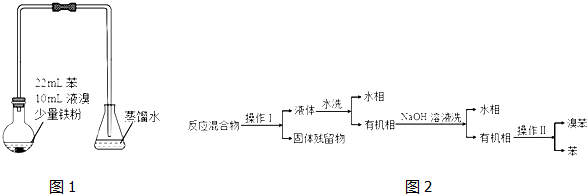
��2����ˮϴ������NaOH��Һϴ����Ҫ�õ��IJ��������Ƿ�Һ©�����ձ���
��3����ˮϴ����ҪĿ���dz�ȥFeBr3����NaOH��Һϴ����ҪĿ���dz�ȥBr2��
��4����ƿ������ˮ��Ƶ�ԭ�����ܽ��˴���ƿ�лӷ������壮
��5����֪�����巢������ȡ����Ӧ���ƲⷴӦ����ƿ��Һ�庬�е����ִ���������Br-��H+�������ʵ�鷽����֤����Ʋ⣮����ѡ�Լ���þ�������Ȼ�̼����ˮ����ˮ������ˮ��
| ��� | ʵ�鲽�� | Ԥ������ | ���� |
| 1 | ����ƿ��Һ��ת���Һ©���������������Ȼ�̼�����Һ���ֱ�ȡ�����ϲ���ɫ��Һ���Թ�A��B�� | ||
| 2 | ��ƿ��Һ�庬���� Br- | ||
| 3 | ��ƿ��Һ�庬���� H+ |