��Ŀ����
10����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ ��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ����1��CԪ�������ڱ��е�λ�õ������ڵڢ�A�壮
��2���õ���ʽ��ʾA����̬�⻯����γɹ��̣�
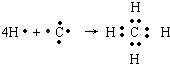 ��
����3��A�ĵ��ʺ�B�ĵ��ʳ�ַ�Ӧ���ɻ�����Ľṹʽ��O=C=O��
��4������Ԫ���У�����������ˮ������������ᣬ�������ڼ������NaOH��Һ�Ļ�ѧ����ʽΪ��Al��OH��3+NaOH=NaAlO2+2H2O��
���� Bԭ��L��ĵ���������K���3����B��ԭ��ԭ�Ӻ������������ֱ�Ϊ2��6����BΪOԪ�أ�A�ĵ���ʽΪ ��A��B��ͬһ���ڣ���AΪCԪ�أ�0.1molC�����ܴ������û���2.24L��������״�������������ʵ���Ϊ0.1mol����Ӧ��C�Ļ��ϼ�Ϊ$\frac{0.1mol��2}{0.1mol}$=+2����Cԭ���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DΪAlԪ�أ��ݴ˽��
��A��B��ͬһ���ڣ���AΪCԪ�أ�0.1molC�����ܴ������û���2.24L��������״�������������ʵ���Ϊ0.1mol����Ӧ��C�Ļ��ϼ�Ϊ$\frac{0.1mol��2}{0.1mol}$=+2����Cԭ���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DΪAlԪ�أ��ݴ˽��
��� �⣺Bԭ��L��ĵ���������K���3����B��ԭ��ԭ�Ӻ������������ֱ�Ϊ2��6����BΪOԪ�أ�A�ĵ���ʽΪ ��A��B��ͬһ���ڣ���AΪCԪ�أ�0.1molC�����ܴ������û���2.24L��������״�������������ʵ���Ϊ0.1mol����Ӧ��C�Ļ��ϼ�Ϊ$\frac{0.1mol��2}{0.1mol}$=+2����Cԭ���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DΪAlԪ�أ�
��A��B��ͬһ���ڣ���AΪCԪ�أ�0.1molC�����ܴ������û���2.24L��������״�������������ʵ���Ϊ0.1mol����Ӧ��C�Ļ��ϼ�Ϊ$\frac{0.1mol��2}{0.1mol}$=+2����Cԭ���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DΪAlԪ�أ�
��1��CΪMgԪ�أ������ڱ��е�λ�ã��������ڵڢ�A�壬
�ʴ�Ϊ���������ڵڢ�A�壻
��2��A����̬�⻯��ΪCH4���õ���ʽ��ʾ�γɹ��̣�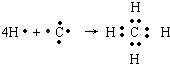 ��
��
�ʴ�Ϊ��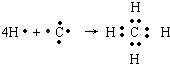 ��
��
��3��A�ĵ��ʺ�B�ĵ��ʳ�ַ�Ӧ���ɻ�����ΪCO2���ṹʽ��O=C=O��
�ʴ�Ϊ��O=C=O��
��4������Ԫ���У�����������ˮ������������ᣬ�������ڼ������ΪAl��OH��3��������NaOH��Һ�Ļ�ѧ����ʽΪ��Al��OH��3+NaOH=NaAlO2+2H2O��
�ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
���� ���⿼��Ԫ��λ�ýṹ���ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ�ע�������õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 53������ϵ�д�
53������ϵ�д��ɺ���$\stackrel{����}{��}$������$\stackrel{ˮ}{��}$ˮ��Һ$\stackrel{����}{��}$$\stackrel{A}{��}$$\stackrel{B}{��}$���ⵥ��
�����й���������ȷ���ǣ�������
| A�� | ��Ԫ���ں�ˮ��û������̬���� | |
| B�� | ����ȡ�Ĺ������õ�������������H2O2 | |
| C�� | ����A����ȡ | |
| D�� | B���ᴿ�������������� |
| A�� | �������ӣ�����������Ų���ȫ��ͬ�����仯ѧ����һ����ͬ | |
| B�� | ����ԭ���γɵ����ӣ�һ������ϡ������Ԫ��ԭ�ӵĺ�������Ų� | |
| C�� | ��ԭ�ӣ������������Ų���ͬ����һ������ͬ��Ԫ�� | |
| D�� | �����ӵĺ�������Ų�һ�������ԭ������С��ϡ������Ԫ��ԭ�ӵĺ�������Ų���ͬ |
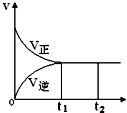 ���淴ӦN2��g��+3H2��g��?2NH3��g��������ӦΪ���ȷ�Ӧ����һ�ܱ������н��У���ͼ�Ǹù����еķ�Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ���ߣ���������������ǣ�������
���淴ӦN2��g��+3H2��g��?2NH3��g��������ӦΪ���ȷ�Ӧ����һ�ܱ������н��У���ͼ�Ǹù����еķ�Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ���ߣ���������������ǣ�������| A�� | t1��t2�������ʵ�Ũ�Ȳ��ٷ����仯 | |
| B�� | t2ʱ�������¶ȣ���Ӧ��ת���ʲ���ı� | |
| C�� | t2ʱ������N2��Ũ�ȣ������H2��ת���� | |
| D�� | ʹ�ô����ɼӿ췴Ӧ���ʣ��������Ч�� |
| A�� | 2.3 g Na��������O2��ȫ��Ӧ������3.4g�������ת�Ƶ�����Ϊ0.1NA | |
| B�� | ��⾫��ͭʱ������������64g����ʱת�Ƶĵ�����Ϊ2NA | |
| C�� | 46 g��NO2��46 g��N2O4���е�ԭ������ͬ | |
| D�� | ��״���£�11.2 L���к���̼̼˫����ĿΪ3NA |