��Ŀ����
����Ŀ���⼰�仯�����������������о�����Ҫ���á��밴Ҫ��ش���������:
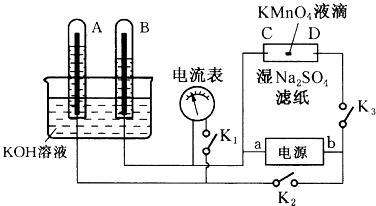
��1�������ҽ�ȡҺ�еĵ�Ԫ����I-��ʽ���ڡ������������Լ���MnO2��ϡ���ᡢ������Һ�����л�ȡ���ʵ⡣�밴Ҫ�������±�:
��� | ��ѡ�Լ� | ��Ӧԭ������������ |
����1 | MnO2��ϡ���� | ���ӷ���ʽ��________ |
����2 | ϡ���ᡢ������Һ | ��Һ������ԭ�������ӷ���ʽ����: ____________ |
��2����Ӧ2HI(g) ![]() H2(g)+I2(g)�������仯��ͼ1��ʾ:����������ͬ��1molHI�ڲ�ͬ�¶ȷֽ��ƽ��ʱ�������ϵ��n(I2)���¶ȱ仯��������ͼ2��ʾ��
H2(g)+I2(g)�������仯��ͼ1��ʾ:����������ͬ��1molHI�ڲ�ͬ�¶ȷֽ��ƽ��ʱ�������ϵ��n(I2)���¶ȱ仯��������ͼ2��ʾ��
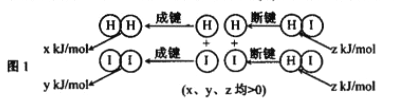
�ٱȽ�2z______(x+y)(�� ��<"����>������=��).
��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1/9,��ƽ��ʱ��HI��ת����=___________��
��ֻ�ı�÷�Ӧ��һ����������д�������HIת���ʵ������ʩ��__________��_________��
��3����֪:i.�ֽ�1molH2O2�ų�����98kJ����.������I-����Һ�У�H2O2�ķֽ��Ϊ: H2O2+I-![]() H2O+IO-����H2O2+IO-
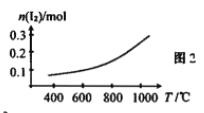
H2O+IO-����H2O2+IO-![]() H2O+O2+I-�졣��.H2O2�ֽ������ܶ�������Ӱ�죬ʵ����ij�¶�ʱ��ͬ������H2OŨ����ʱ��ı仯��ͼ3��4��ʾ:
H2O+O2+I-�졣��.H2O2�ֽ������ܶ�������Ӱ�죬ʵ����ij�¶�ʱ��ͬ������H2OŨ����ʱ��ı仯��ͼ3��4��ʾ:

��������������Ϣ�ɵõ������½���:
��H2O2�ֽⷴӦ���Ȼ�ѧ����ʽΪ___________��
��H2O2�ķֽ�������_________�йء�
��������Mn2+����ʱ����Һ����ԽǿH2O2�ֽ�����Խ�����Ľ����Ƿ���ȷ_______(������"��������)��c(Mn2+)��H2O�ֽ����ʵ�Ӱ����__________��
���𰸡� MnO2+4H++2I- =Mn2++I2+2H2O O2+4H++4I-=2I2+2H2O > 40% ����I2 ���� 2H2O2(1) =2H2O(1) + O2(g) ��H=-196 kJ/mol c(I-)��c(Mn2+)����ҺpH �� c(Mn2+)Խ��H2O2�ֽ�����Խ��
��������(1).�����������£��������̰ѵ���������Ϊ�ⵥ�ʣ�������ԭΪMn2+����ȷ��Ϊ��
MnO2+4H++2I- =Mn2++I2+2H2O�����������������ӱ������е���������Ϊ�ⵥ�ʣ���ȷ��Ϊ��O2+4H++4I-=2I2+2H2O��
��2���ٸ���ͼʾ��֪���¶����ߣ���������÷�ӦΪ���ȷ�Ӧ�����Է�Ӧ��������С����������������2z> (x+y)����ȷ����>
�� 2HI(g) ![]() H2(g) + I2(g) �������������Ϊ1L
H2(g) + I2(g) �������������Ϊ1L
��ʼŨ�� 1 0 0
�仯Ũ�� 2x x x
ƽ��Ũ�� 1-2x x x
����ƽ�ⳣ�����㣺x2/(1-2x)2= K=1/9�����x=0.2mol/L
HI��ת����=2��0.2��1��100%=40%����ȷ�𰸣�40%��
�������������䣬�����������������Ũ�ȣ������¶ȶ��������HIת���ʣ���ȷ�𰸣�����I2 �� ���£�
��3����H2O2�ķֽ����Ϊ��H2O2+I-![]() H2O+IO-����H2O2+IO-
H2O+IO-����H2O2+IO-![]() H2O+O2+I-�졣����ʽ����Ӵ��������ΪH2O2�ֽⷴӦ�Ļ�ѧ����ʽ��Ȼ����ݷֽ�1molH2O2�ų�����98kJ ���Ϳ���д���Ȼ�ѧ����ʽ����ȷ��2H2O2(1) =2H2O(1) + O2(g) ��H=-196 kJ/mol��
H2O+O2+I-�졣����ʽ����Ӵ��������ΪH2O2�ֽⷴӦ�Ļ�ѧ����ʽ��Ȼ����ݷֽ�1molH2O2�ų�����98kJ ���Ϳ���д���Ȼ�ѧ����ʽ����ȷ��2H2O2(1) =2H2O(1) + O2(g) ��H=-196 kJ/mol��
�ڸ���2��ͼ��������H2O2�ķֽ�������c(Mn2+)��c(I-)����ҺpH���й�����ȷ����c(Mn2+)��c(I-)����ҺpH��
������Mn2+����ʱ������ͼ��3���Կ�������Һ�ļ���ԽС��H2O2�ֽ�����Խ����ȷ�𰸣�����ͼ��4���������Բ��������£�c(Mn2+)Խ��H2O2�ֽ�����Խ����ȷ�𰸣�c(Mn2+)Խ��H2O2�ֽ�����Խ��

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�