��Ŀ����
11���±���Ԫ�����ڱ���һ���֣���ش��й����⣺| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
 ��
����2���������γ��������������Ԫ����������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ2Al+2KOH+2H2O�T2KAlO2+3H2����
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������ΪKOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4���û�ѧʽ��ʾ����
��4����Ԫ�����Ԫ�����ߺ˵����֮����26��
��5����д���ڵ��⻯����������Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��6����д����Ԫ�ص�����������ˮ�������Ԫ�ص�����������ˮ���ﷴӦ�����ӷ���ʽAl��OH��3+3H+�TAl3++3H2O��
���� ����Ԫ�������ڱ��е�λ��֪���١�����Ԫ�طֱ���C��N��F��Mg��Al��S��Cl��Ar��K��BrԪ�أ�
��1����ЩԪ��������õ�Ԫ����Ar����ԭ�Ӻ�����18�����ӣ�������3�����Ӳ㣻
��2���������γ��������������Ԫ��������������������ˮ������KOH������KOH��Һ��Ӧ����ƫ����غ�������
��3��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��
��4����Ԫ�����Ԫ�����ߺ˵����֮��=35-9��
��5���ڵ��⻯���ǰ�������������������NO��ˮ��
��6����Ԫ�ص�����������ˮ������������������Ԫ�ص�����������ˮ�����Ǹ����ᣬ���߷�Ӧ���ɸ���������ˮ��
��� �⣺����Ԫ�������ڱ��е�λ��֪���١�����Ԫ�طֱ���C��N��F��Mg��Al��S��Cl��Ar��K��BrԪ�أ�
��1����ЩԪ��������õ�Ԫ����Ar����ԭ�Ӻ�����18�����ӣ�������3�����Ӳ㣬��ԭ�ӽṹʾ��ͼΪ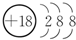 ��
��
�ʴ�Ϊ��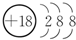 ��
��
��2���������γ��������������Ԫ��������������������ˮ������KOH������KOH��Һ��Ӧ����ƫ����غ���������Ӧ����ʽΪ2Al+2KOH+2H2O�T2KAlO2+3H2����
�ʴ�Ϊ������2Al+2KOH+2H2O�T2KAlO2+3H2����
��3��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��������ǿ��˳����K��Mg��Al���ǽ�����ǿ��˳����Cl��S��C���������Լ���������ǿ��˳������ΪKOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
�ʴ�Ϊ��KOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
��4����Ԫ�����Ԫ�����ߺ˵����֮��=35-9=26���ʴ�Ϊ��26��
��5���ڵ��⻯���ǰ�������������������NO��ˮ����Ӧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��6����Ԫ�ص�����������ˮ������������������Ԫ�ص�����������ˮ�����Ǹ����ᣬ���߷�Ӧ���ɸ���������ˮ�����ӷ���ʽΪAl��OH��3+3H+�TAl3++3H2O���ʴ�Ϊ��Al��OH��3+3H+�TAl3++3H2O��
���� ���⿼��Ԫ�����ڱ��ṹ��Ԫ���������ۺ�Ӧ�ã�Ϊ��Ƶ���㣬��ȷ�������ʡ�ԭ�ӽṹ��Ԫ���������ں����ɽ��ע���������������ԣ���Ŀ�ѶȲ���

| A�� | 1molCH3+��̼�����ӣ��к��е�����Ϊ10NA | |
| B�� | 1molͭ��������������Ӧ��ת�Ƶ�����Ϊ2NA | |
| C�� | �ö��Ե缫���CuCl2��Һ����������32gͭʱ����·��ͨ���ĵ�����ΪNA | |
| D�� | 0.84g NaHCO3�����������Ӻ�����������Ϊ0.03NA |
| A�� | ��ͳ���ǽ���������ָ�����ά��������ˮ�ࡢ�մɵȹ����β��� | |
| B�� | �������ǽ���������Ȼ�˷��˴�ͳ���ǽ������ϵ�ȱ�㣬��ǿ�ȱȽϲ� | |
| C�� | ���½ṹ���Ͼ������¡�����ʴ��Ӳ�ȴ���ĥ���ܶ�С���ŵ� | |
| D�� | ��ͳ���ǽ������Ϻ��������ǽ������ϵ���Ҫ�ɷֶ��ǹ����� |

��1��д��D�����й����ŵĽṹʽ
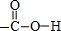
��2���١���Ӧ������ȡ����Ӧ���Ǣ�
��3��д���ں͢߷�Ӧ�Ļ�ѧ����ʽ
��CH2BrCH2Br+2NaOH$\stackrel{��}{��}$HOCH2CH2OH+2NaBr��CaC2+2H2O=Ca��OH��2+C2H2��
II����������ķ�Ӧ·��������Ϣ�ش�4��-��7���ĸ�С��
X$��_{��}^{Cl_{2}������}$
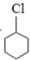 $\stackrel{��}{��}$
$\stackrel{��}{��}$ $\stackrel{��}{��}$Y$\stackrel{��}{��}$
$\stackrel{��}{��}$Y$\stackrel{��}{��}$
��4���밴Ҫ����д���б�����δ�����ߵ����пհ�
| ��Ӧ�� | ��Ӧ�� | ��Ӧ�� | |
| �Լ������� | |||
| ��Ӧ���� |
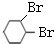 +2NaOH $��_{��}^{��}$
+2NaOH $��_{��}^{��}$ 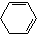 +2NaBr+2H2O
+2NaBr+2H2O��֪��
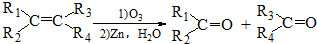 ��R1��R2��R3��R4������H��
��R1��R2��R3��R4Ϊ������H����6������������Ϣ���M�������Ļ�ѧ����ʽ����Ҫ����ƽ���л�����Ҫдȫ����
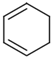 $��_{��2��Zn��H_{2}O}^{��1��O_{3}}$OHC-CH2CH2CHO+OHC-CHO�������к��������ŵ�������ȩ����
$��_{��2��Zn��H_{2}O}^{��1��O_{3}}$OHC-CH2CH2CHO+OHC-CHO�������к��������ŵ�������ȩ������7��д���������������������л���Ľṹ��ʽCH3CH2C��CCH=CH2��CH3C��CCH2CH=CH2��
����M��Ϊͬ���칹�� ������֬���� �۷��ӽṹ����֧�� �ܺ˴Ź���������ʾ���ĸ����ҷ����֮��Ϊ1��2��2��3��
| A�� | SO3��HCHO | B�� | BF3��NH3 | C�� | BeCl2��SCl2 | D�� | H2O��SO2 |
| A�� | ﮣ�Li����ˮ��Ӧ������ˮ��Ӧ���� | |
| B�� | ����At��Ϊ��ɫ���壬AgAt������ˮҲ������ϡ���� | |
| C�� | �������У�﨣�Rb����ȼ�ղ�����Ƶ�ȼ�ղ�������� | |
| D�� | HBr���ȶ��Ա�HIǿ |
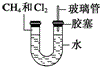 ��ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ�е��ܽ�ȿ��Ժ��ԣ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ط�Ӧһ��ʱ�䣮
��ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ�е��ܽ�ȿ��Ժ��ԣ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ط�Ӧһ��ʱ�䣮