��Ŀ����
1��I����ͼ1Ϊ��25mL 0.1mol/L NaOH��Һ����εμ�0.2mol/LCH3COOH��Һ��������ҺpH�ı仯���ߣ���ش�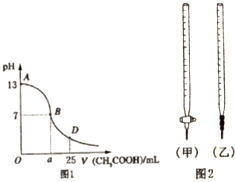
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ����ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�AB���䣨����ȷ�����ʲ��𣩣�
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ��A��ѡ����ĸ����
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ��̪ | ���ף� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ʯ�� | ���ң� |
| D | �� | �� | ʯ�� | ���ң� |
A��c��OH-������c��CH3COO-��B��c��OH-����c��CH3COO-��
C��c��OH-������c��CH3COO-��D�������������������
��4����D��ʱ����Һ��c��CH3COO-��+c��CH3COOH��=2c��Na+�����������������=������
II��t��ʱ��ijϡ������Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=13��
��5�����¶���ˮ�����ӻ�����KW����ֵΪ10-13��
��6�����¶��£�t�棩����100mL 0.1mol/L��ϡH2SO4��100mL 0.4mol/L��NaOH��Һ��ϣ���Һ����仯���Բ��ƣ�����Һ��pH=12��
���� ��1��NaOH��CH3COOHǡ����ȫ��Ӧ�����ɴ����ƣ�������Ϊǿ�������Σ�ˮ�����Һ�Լ��ԣ�pH��7��
��2�����ݱ�ʵ������ζ����ʽ�ζ�������ʢ���ᣬ��ʽ�ζ�������ʢ�żǿ��ζ�����ʱӦѡ�÷�̪��ָʾ����������
��3����AB�����ڣ�����CH3COOH��NaOHǡ����ȫ��Ӧ�Լ�CH3COOH���㣬����������Һ������Һ�Լ������ֿ����ԣ�
��4����D��ʱ��NaOH��CH3COOH��Ӧ��ʣ��CH3COOH����Һ�����Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ���
��5������ˮ�����ӻ�����Kw=c��H+��•c��OH-�������㣻
��6�����ݼ���ó���Ӧ�����������ݷ�Ӧ���ʣ��ļ�����ʵ��������c��OH-����Ȼ�����ˮ�����ӻ���������c��H+����������pH��
��� �⣺��1��NaOH��CH3COOHǡ����ȫ��Ӧ��NaOH+CH3COOH=CH3COONa+H20�����ɵĴ�����Ϊǿ�������Σ���Һ�Լ��ԣ�pH��7������AB֮�䣬
�ʴ�Ϊ����AB��
��2��A����ζ����ʢ������ʽ�ζ����У�ָʾ��ѡ�÷�̪����A��ȷ��
B����ζ��ᣬ��ʢ������ʽ�ζ����У��ζ���Ӧ��ѡ���ң���B����
C����ζ����ʢ���ڼ�ʽ�ζ����У��ζ���Ӧ��ѡ�üף���C����
D����ζ��ᣬ��ʢ���ڼ�ʽ�ζ����У�ָʾ��ѡ�÷�̪����D����
�ʴ�Ϊ��A��
��3����AB�����ڣ�c��OH-����c��H-����˵����Һ�Լ��ԣ���NaOH��CH3COOHǡ�÷�Ӧʱ���Լ��ԣ���ʱ���ɵ���Һ����Ϊ�����ƣ�c��OH-��С��c��CH3COO-������NaOH��CH3COOH��Ӧ��ʣ��NaOH����Һ��Ȼ�Լ��ԣ���ʱ��ʣ���NaOH���ܴ���c��OH-������c��CH3COO-����Ҳ�п���ʣ���NaOH��CH3COONa��CH3COO-ˮ��֮��ʣ���CH3COO-��Ũ����ȣ�����D��ȷ��
�ʴ�Ϊ��D��
��4����D��ʱ����Ӧ��CH3COOHʣ�࣬��Һ�����Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ������������غ㣬��ʱ��c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ��=��
��5��ˮ�����ӻ�����Kw=c��H+��•c��OH-��=10-a��10-b=10-��a+b��=10-13��
�ʴ�Ϊ��10-13��
��6��ϡH2SO4��Һ��NaOH��Ӧ�Ĺ�ϵʽ��H2SO4��2NaOH��
1 2
100mL��0.1mol•L-1 100mL��0.4mol•L-1
������������ļ�Ϊ��0.02mol����c��OH-��=$\frac{0.02mol}{0.2L}$=0.1mol•L-1��
����c��H+��=$\frac{1{0}^{-13}}{0.1}$=10-12mol•L-1������Һ��pH=12��
�ʴ�Ϊ��12��
���� ���⿼��������ϵĶ����жϡ�����к͵ζ���֪ʶ����Ŀ�Ѷ��еȣ�ע����������϶����жϼ���ҺpH�ļ��㷽������ȷ�к͵ζ�����������������ѧ���ķ�����������ѧʵ�顢��ѧ����������

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�| A�� | $\frac{1}{7}$��1-a�� | B�� | $\frac{3}{4}$a | C�� | $\frac{6}{7}$ ��1-a�� | D�� | $\frac{12}{13}$ ��1-a�� |
| A�� | ��ϩ��ʵ��ʽC2H4 | B�� | �Ҵ��Ľṹ��ʽC2H6O | ||
| C�� | ���Ȼ�̼�ĵ���ʽ��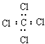 | D�� | 3��3��4-��������ķ���ʽC9H20 |
| A�� | ��CuSO4��Һ�м����������� | |
| B�� | ��Na2SiO3��Һ��ͨ�����CO2���� | |
| C�� | �����ʵ�����NaHCO3��Na2O2����ˮ | |
| D�� | �����ʵ���Ũ�ȡ��������FeCl3��KI��Һ��� |
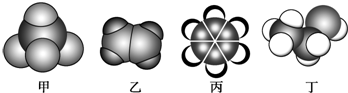
| A�� | ����ʹ���Ը��������Һ��ɫ | |
| B�� | �ҿ�����ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ | |
| C�� | ������ˮ���Է���ȡ����Ӧ | |
| D�� | ����ϡ���������¿������ᷢ��ȡ����Ӧ |
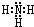 ��
��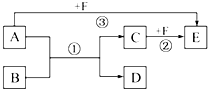 ��֪��A��B��C��D��E��F����ѧ��ѧ�̲������ֳ��������ʣ�����֮������ͼ��ʾ���ת����ϵ����Ӧ���������ֲ���δ���������ش��������⣺
��֪��A��B��C��D��E��F����ѧ��ѧ�̲������ֳ��������ʣ�����֮������ͼ��ʾ���ת����ϵ����Ӧ���������ֲ���δ���������ش��������⣺