��Ŀ����
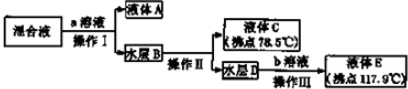
13��ʵ�����о���ѧ�Ļ������Իش��������⣺��1��ij�о���ѧϰС���Ա�ֱ��������ͼ�ļס��ҡ�����������ʵ��װ����ȡ������������ش��������⣻����A�Թ��л���Ҵ���Ũ����IJ����������Թ�A�м����Ҵ���Ȼ���������Թ�ע��Ũ���ᣬ�ӱ����Թܣ�
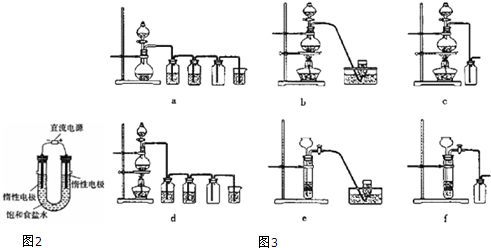
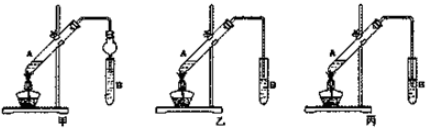
�ڼס��ҡ�������װ���У�����ѡ�õ�װ���DZ���ѡ��ס������ҡ�����������
�������������к��н϶��Ҵ������ᣬijͬѧ�����ͼ��������������ʣ�
��������Ҫ�����Һת�Ƶ���Һ©�������н��в����������������������b���������ᣮ
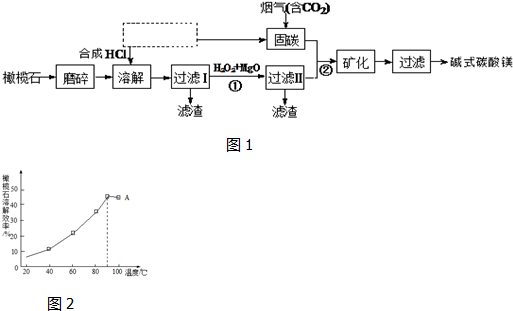
��2����ͼΪ��ʦ������ʾʯ���ͷֽ�ʵ���װ�ã�
ʵ��1������ʯ���͵Ŀ���ʯ����Ӳ�ʲ����ܵײ����Թ��м�����ʯƬ�������Ƭ��ǿ�ȣ�ʯ��������ͨ�����ȵ���ʯƬ���淢����Ӧ������һ���������壮
����2�������ɵ�����ͨ�����Ը��������Һ��
����3�������ɵ�����ͨ��������Ȼ�̼��Һ��
����4���ڵ��ܿڵ�ȼ���ɵ����壮
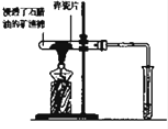
��װ�������Ƭ�������Ƿ�ֹҺ�屩�У�ʵ��4��ȼ����ǰ������еIJ����Ǽ������崿�ȣ�
��ʵ��2�����Ը��������Һ��ɫ��˵�����ɵ������к��в������������������������������������
���о�������ʯ���ͷֽ�IJ����к���ϩ������������֤�����ɵ������к�����������Ը�ʵ��ĸĽ������ǽ����ɵ�����ͨ��ʢ�����Ը�����ص���Һ��ϴ��ƿ���ȼ�����������Ļ����ҷų��������ȣ���Ҫ˵��ʵ��������õ���ʵ��ҩƷ���۲쵽��ʵ������
���� ��1������ȡ�����������˷�Ӧ���Ҵ��������⣬����Ũ��������������ˮ����������Һ�ܶȴ�С�жϼ����Լ���˳��
�ڳ����ܲ����쵽B�Թ�Һ���£���ֹ�����Һ��������ȷ�Ӧ����Թ��У�
���Ʊ���������ʱ���ñ���̼������Һ����������������Ҫ���������������������ڱ���̼���ƣ��������������ܽ�ȣ����ڷֲ㣬������ȡ��Һ�ķ������������Ҵ���ˮ���ܣ������ܱ�̼�������գ����ڳ�ȥ���ʣ�
��2����װ��ͼ������֪�������Ƭ�Ǵ����ã����Ƭ�д��ͻ����������ÿ�ȼ�������ȼ��Ҫ�������崿�ȣ�
�ڲ������������������Һ������ɫ��
�����ø��������Һ����ϩ����������ȼ�չ۲����������ƣ�
��� �⣺��1������ȡ����������Ũ������������A�Թ��е�Һ̬���������ᡢ�Ҵ���Ũ���ᣬ�����Լ�Ӧ�ȼ����ܶ�С���ټ����ܶȴ���Լ�����A�Թ��л���Ҵ���Ũ����IJ����ǣ������Թ�A�м����Ҵ���Ȼ���������Թ�ע��Ũ���ᣬ�ӱ����Թܣ�
�ʴ�Ϊ�������Թ�A�м����Ҵ���Ȼ���������Թ�ע��Ũ���ᣬ�ӱ����Թܣ�
�ڳ����ܲ����쵽B�Թ�Һ���£���ֹB�Թ�����Һ������A�Թ��У���װ��B�Թ��е�������Һ���£�������������
�ʴ�Ϊ������
�۲�������Ҫ�����Һת�Ƶ���Һ©������ȡ��Һ��������������õ��Ҵ���ˮ��Ϊ�����ƣ�����������Һ��Һ�õ����ᣬ����õ����ᣬ
�ʴ�Ϊ����Һ©�����������
��2���ټ���ʯ����ʱ�������Ƭ��ʯ���ͷֽ�ϻ������������Ƭ�ܼӿ췴Ӧ���ʣ����Ƭ�����������������������Ӷ��ٽ�ʯ���ͷֽ⣬�������ã���ȼ����ǰ������еIJ����Ǽ������崿�ȣ�
�ʴ�Ϊ���������������崿�ȣ�
�ڲ������������������Һ������ɫ������������ʹ����ɫ��
�ʴ�Ϊ������������
��ʯ���ͷֽ�IJ����к���ϩ������������֤�����ɵ������к���������ʵ�����Ϊ�������ɵ�����ͨ��ʢ�����Ը�����ص���Һ��ϴ��ƿ���ȼ�����������Ļ����ҷų��������ȣ�
�ʴ�Ϊ�������ɵ�����ͨ��ʢ�����Ը�����ص���Һ��ϴ��ƿ���ȼ�����������Ļ����ҷų��������ȣ�
���� ���⿼����������������ȡ����Ŀ�Ѷ��еȣ�ע������������������ȡԭ����װ��ѡ����ȷ��Ӧ�б���̼������Һ�����ü��������������ĵ��ܵ���ȷ����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�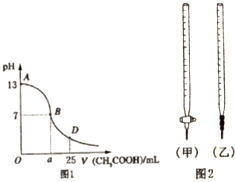
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ����ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�AB���䣨����ȷ�����ʲ��𣩣�
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ��A��ѡ����ĸ����
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ��̪ | ���ף� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ʯ�� | ���ң� |
| D | �� | �� | ʯ�� | ���ң� |
A��c��OH-������c��CH3COO-��B��c��OH-����c��CH3COO-��
C��c��OH-������c��CH3COO-��D�������������������
��4����D��ʱ����Һ��c��CH3COO-��+c��CH3COOH��=2c��Na+�����������������=������
II��t��ʱ��ijϡ������Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=13��
��5�����¶���ˮ�����ӻ�����KW����ֵΪ10-13��
��6�����¶��£�t�棩����100mL 0.1mol/L��ϡH2SO4��100mL 0.4mol/L��NaOH��Һ��ϣ���Һ����仯���Բ��ƣ�����Һ��pH=12��
| A�� | �����������ڹ��յ�����������һ�ȼ���ķ�Ӧ | |
| B�� | ��ϩ��������Ȼ�̼��Һ����������ķ�Ӧ | |
| C�� | ��ϩ��ˮ�����Ҵ��ķ�Ӧ | |
| D�� | ��ϩ�������ɾ���ϩ�ķ�Ӧ |
| A�� | ��ϩ�Ľṹ��ʽCH2CH2 | B�� | Na2O2����Ԫ�صĻ��ϼ�Ϊ-2 | ||
| C�� | Cl-�Ľṹʾ��ͼ�� | D�� | ��������ʽ��CH2O |
| A�� | ��ѧ��Ӧ�ﵽ��ѧƽ��״̬�����淴Ӧ������ȣ���ָͬһ���ʵ��������ʺ�����������ȣ����ò�ͬ���ʱ�ʾʱ����Ӧ���ʲ�һ����� | |
| B�� | ��״���£�1L������ȫȼ������CO28L | |
| C�� | 2.4gMg������O2������N2��ȫ��Ӧ��ת�Ƶ���������0.2NA | |
| D�� | 1L 1mol•L-1 CH3COOH��Һ�У�����CH3COO-��CH3COOH������ΪNA |
��1��������ijͬѧ���й����ʽ��з�����б���
| �� | �� | �� | ���������� | ���������� | |
| ��һ�� | Na2CO3 | H2SO4 | NaHCO3 | CaO | CO2 |
| �ڶ��� | NaOH | HCl | NaCl | Na2O | CO |
| ������ | NaOH | CH3COOH | CaF2 | Al2O3 | SO2 |
��2���������Һ�ı��������Ƿ�ɢ����ֱ����С�����������Һ�����õķ����ǹ۲��Ƿ��ܷ������������
��3������3����Ӧ����Ҫ����д�������
��2Na2O2+2H2O�T4NaOH+O2����Ӧ�У�ÿ����1mol Na2O2����16g O2��
��2NaHCO3�TNa2CO3+H2O+CO2����Ӧ�У�ÿ����168g NaHCO3�����������22.4L CO2��
��Cl2+H2O�THCl+HClO��Ӧ�У������ÿ����22.4L Cl2��ת��1mol���ӣ�
��4����һ���ܱ������з���M��N��Q��P�������ʣ���һ�������·�����ѧ��Ӧ��һ��ʱ�����й��������±�����Ҫ��ش��������⣺
| ���� | M | N | Q | P |
| ��Ӧǰ������g�� | 50 | 1 | 3 | 12 |
| ��Ӧ��������g�� | X | 26 | 3 | 30 |
| A�� | ��S��0 | B�� | ��S��0 | C�� | ��S=0 | D�� | ��ȷ�� |