��Ŀ����
1���±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�
�Իش��������⣺
��1���뻭��Ԫ��D�Ļ�̬ԭ�ӵļ۵����Ų�ͼ
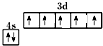 ����Dͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������Dԭ����ͬ��Ԫ����8�֣�
����Dͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������Dԭ����ͬ��Ԫ����8�֣���2��A������⻯�������ԭ�ӵ��ӻ���ʽΪsp3��C�ڿ�����ȼ�ղ���ķ��ӹ���ΪV�Σ����以Ϊ�ȵ�����ĵ��ʵķ���ʽΪO3��
��3��B���ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
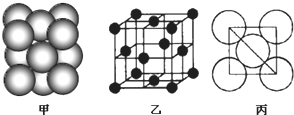
?������Bԭ�ӵ���λ��Ϊ12����ѻ���ʽ�ǣ���ǻ�����öѻ���?��Bԭ�ӵİ뾶Ϊd cm����NA��ʾ�����ӵ�������M��ʾBԭ�ӵ����ԭ����������þ�����ܶ�Ϊ$\frac{\sqrt{2}M}{8{N}_{A}{d}^{3}}$g/cm3������ĸ��ʾ����
���� ����Ԫ�������ڱ��е�λ��֪��A��B��C��D�ֱ���C��Al��S��MnԪ�أ�
��1��Mnԭ��3d��4s�ܼ��Ϸֱ���5��2�����ӣ���Dͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������Dԭ����ͬ��Ԫ����Ca��Sc��Ti��V��Fe��Co��Ni��Zn��
��2��A������⻯���Ǽ��飬���������Cԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�
S�ڿ�����ȼ�ղ����Ƕ������������������Sԭ�Ӽ۲���ӶԸ�����3�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж϶�������ķ��ӹ��ͣ�ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
��3��������Alԭ�ӵ���λ��=3��8��2����ѻ���ʽ�����öѻ���Al��ԭ�Ӱ뾶Ϊdcm������ͼ֪��ÿ����Խ�����3��ԭ�ӹ��ߣ����߳�=2$\sqrt{2}$dcm���������=��2$\sqrt{2}$d��3cm3?�þ�����ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ܶ�=$\frac{\frac{M}{{N}_{A}}��4}{V}$��
��� �⣺����Ԫ�������ڱ��е�λ��֪��A��B��C��D�ֱ���C��Al��S��MnԪ�أ�
��1��Mnԭ��3d��4s�ܼ��Ϸֱ���5��2�����ӣ���۵����Ų�ͼΪ ����Dͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������Dԭ����ͬ��Ԫ����Ca��Sc��Ti��V��Fe��Co��Ni��Zn��������8�֣�
����Dͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������Dԭ����ͬ��Ԫ����Ca��Sc��Ti��V��Fe��Co��Ni��Zn��������8�֣�
�ʴ�Ϊ�� ��8��
��8��
��2��CԪ�ص�����⻯���Ǽ��飬���������Cԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ�������֪Cԭ���ӻ�����Ϊsp3��
S�ڿ�����ȼ�ղ����Ƕ������������������Sԭ�Ӽ۲���ӶԸ�����3�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ������۵ö�������ķ��ӹ���ΪV�Σ�ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壬���以Ϊ�ȵ�����ĵ��ʵķ���ʽΪO3��
�ʴ�Ϊ��sp3��V��O3��
��3��������Alԭ�ӵ���λ��=3��8��2=12����ѻ���ʽ�����öѻ���Al��ԭ�Ӱ뾶Ϊdcm������ͼ֪��ÿ����Խ�����3��ԭ�ӹ��ߣ����߳�=2$\sqrt{2}$dcm���������=��2$\sqrt{2}$d��3cm3?�þ�����ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ܶ�=$\frac{\frac{M}{{N}_{A}}��4}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{��2\sqrt{2}d��^{3}}$g/cm3=$\frac{\sqrt{2}M}{8{N}_{A}{d}^{3}}$g/cm3��
�ʴ�Ϊ��12���ǣ�$\frac{\sqrt{2}M}{8{N}_{A}{d}^{3}}$g/cm3��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ���ӻ���ʽ�жϡ����ռ乹���жϡ��ȵ������֪ʶ�㣬���ü۲���ӶԻ��������ж�ԭ���ӻ���ʽ�����ռ乹�ͣ��ѵ��Ǿ������㣬��Ŀ�Ѷ��еȣ�

| A�� | 11.2 L�����������е�ԭ����ΪNA | |
| B�� | 7.8g��Na2O2�к��е������ӵ���ĿΪ0.2NA | |
| C�� | 0.10mol Fe ��������ˮ������Ӧ���ɵ�H2������Ϊ0.10NA | |
| D�� | 54g Al�ֱ���������ϡ���ἰ����������Һ��Ӧʧȥ�ĵ���������6NA |
| A�� | �������ȵ������� | B�� | �����ǵ�������� | ||
| C�� | ���������õ���չ�� | D�� | �н��������� |
| A�� | Na��K��Rb | B�� | N��P��As | C�� | Si��P��Cl | D�� | O��S��Cl |
| A�� | ��ϩ����Ȳ | B�� | ��ϩ����ϩ | C�� | �������� | D�� | �����ױ� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | �����Ȼ�粒��壬��Һ��$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$��С | |
| B�� | ��ˮ����ϡ�ͣ���Һ����һ����ǿ | |
| C�� | ��ˮϡ�ͣ�ƽ�ⳣ��Kb���� | |
| D�� | ����NaOH���壬ƽ�������ƶ� |
 ���ʵĽṹ�������ʣ����ʷ�ӳ��ṹ�ص㣮
���ʵĽṹ�������ʣ����ʷ�ӳ��ṹ�ص㣮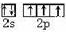 ��
��
