��Ŀ����
����Ŀ����ˮ����ͭ(CuSO4)Ϊ��ɫ��Ұ�ɫ��ĩ����ˮ��Һ����ɫ,�������ԡ�������ɱ�����͵�⾫��ͭʱ�ĵ��Һ��ij��ѧ����С��ͨ���������ͭ���ȷֽ��̽��ʵ�飬�ⶨ�������ijɷ�(��֪����ͭ��ȫ�ֽ⣬����������CuO)��ʵ��װ����ͼ��ʾ,�ش���������

(1)ʵ��ǰ����еIJ�����_________������a��������_____������ʱ�Թ���ڱ�����Ҫ�Ⱦ��ȼ��ȣ��ټ��м��ȣ���Ŀ����__________��
(2)ʵ�����ʱ����f����Ͳ�Ƿ��ռ���ˮ��ȷ���������������_______���ѧʽ����
(3)װ��c��������_________��
(4)װ��d�еĻ�ѧ����ʽΪ____��_____����С���Ա������װ��d����������һ������ܣ���ԭ����_______��
(5)������װ�õ�ʵ�����ǰ��������������£�(�ѧʽ)��
ʵ��ǰ��ˮ����ͭ������/g | ʵ���װ��d���ӵ�����/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
6.4 | 2.56 | 224 |
ͨ�����㣬д��CuSO4��ǿ�ȷֽ�Ļ�ѧ����ʽ��______��
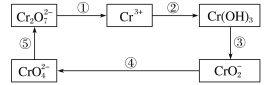
���𰸡����װ�õ������� �ƾ���� ��ֹ�Թ�����ʱ�������ȶ����� O2 �������� CaO��SO2=CaSO3 2NaOH��SO2=Na2SO3+H2O e��ˮ��������d�У��������Ӷ���SO2�Ķ���������ɸ��� 4CuSO4![]() 4CuO+2SO2��+O2��+2SO3��
4CuO+2SO2��+O2��+2SO3��
��������
ʵ�����������ã�����װ�á�����ת��������������������װ�á���ˮ������װ�ã���������ͭ�ֽ���ܷ����������2CuSO4![]() 2CuO+2SO2��+O2����CuSO4
2CuO+2SO2��+O2����CuSO4![]() CuO+SO3������������Ӧͬʱ�������жϡ�
CuO+SO3������������Ӧͬʱ�������жϡ�
(1)Ϊȷ���������������ȷ��ʵ��ǰ����еIJ����Ǽ��װ�õ������ԣ�����a�������Ǿƾ���ƣ�����ʱ�Թ���ڱ�����Ҫ�Ⱦ��ȼ��ȣ��ټ��м��ȣ���Ŀ���Ƿ�ֹ�Թ�����ʱ�������ȶ����ѡ�
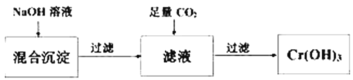
(2)���������ҵķ������Ʊ����壬ͨ��������������������������ͨ��Ũ�������������������������ͨ����ʯ�����ն�������������㣬���������ˮ�������ⶨ�����������ʵ�����ʱ����f����Ͳ�Ƿ��ռ���ˮ��ȷ���������������O2��
(3)װ��c��������Ũ�������������������������ȷ����ʯ�����յ�ֻ�ж�������
(4)װ��d�еĻ�ѧ����ʽΪCaO��SO2=CaSO3��2NaOH��SO2=Na2SO3+H2O��CaO��H2O=Ca(OH)2����С���Ա������װ��d����������һ������ܣ���ԭ����e��ˮ��������d�У��������Ӷ���SO2�Ķ���������ɸ��š�
(5)n��O2��=![]() mol=0.01mol��������ʽ��2CuSO4
mol=0.01mol��������ʽ��2CuSO4![]() 2CuO+2SO2��+O2����Ӧʱ��ֻ����0.02molSO2���ֲd�����ӵ�����2.56g��
2CuO+2SO2��+O2����Ӧʱ��ֻ����0.02molSO2���ֲd�����ӵ�����2.56g��![]() =0.04mol��6.4g����ͭ
=0.04mol��6.4g����ͭ![]() =0.04mol������0.02molSO2������0.02molSO3��ͨ�����㣬д��CuSO4��ǿ�ȷֽ�Ļ�ѧ����ʽ��4CuSO4
=0.04mol������0.02molSO2������0.02molSO3��ͨ�����㣬д��CuSO4��ǿ�ȷֽ�Ļ�ѧ����ʽ��4CuSO4![]() 4CuO+2SO2��+O2��+2SO3����
4CuO+2SO2��+O2��+2SO3����

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�����Ŀ����֪��N2(g)+3H2(g)===2NH3(g) ��H=-92kJ/mol����ʼ��Ӧ��Ϊ![]() ��
��![]() �����ʵ���֮��Ϊ1:3���������ʵ������䣬�ڲ�ͬѹǿ���¶��£���Ӧ�ﵽƽ��ʱ����ϵ��
�����ʵ���֮��Ϊ1:3���������ʵ������䣬�ڲ�ͬѹǿ���¶��£���Ӧ�ﵽƽ��ʱ����ϵ��![]() �����ʵ����������±���
�����ʵ����������±���
�¶� ѹǿ | 400�� | 450�� | 500�� | 600�� |
20MPa | 0.387 | 0.274 | 0.189 | 0.088 |
30MPa | 0.478 | 0.359 | 0.260 | 0.129 |
����˵����ȷ����
A. ��ϵ��![]() �����ʵ�������Խ��������Ӧ����Խ��
�����ʵ�������Խ��������Ӧ����Խ��
B. ��Ӧ�ﵽƽ��ʱ�� ![]() ��
��![]() ��ת���ʾ�Ϊ1
��ת���ʾ�Ϊ1
C. ��Ӧ�ﵽƽ��ʱ���ų���������Ϊ92.4kJ
D. 600����30MPa�·�Ӧ�ﵽƽ��ʱ������![]() �����ʵ������
�����ʵ������