题目内容
【题目】铬及其化合物常被应用于冶金、化工、电镀、制药、纺织等行业,但使用后的废水因其中含高价铬的化合物而毒性很强,必须进行处理。工业上往往采取下列循环工艺防止铬的污染:
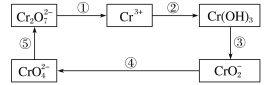
(1)上述各步反应中属于氧化还原反应的是__________(填序号)。
(2)第①步,含![]() 的废水在酸性条件下用绿矾FeSO4·7H2O处理,写出该反应的离子方程式:_________。
的废水在酸性条件下用绿矾FeSO4·7H2O处理,写出该反应的离子方程式:_________。
(3)第②步,向上述反应后的溶液中加入适量的碱石灰,使铬离子转变为Cr(OH)3沉淀。处理后,沉淀物中除了Cr(OH)3外,还有_____、____(写化学式)。已知Cr(OH)3性质类似Al(OH)3,是既能与强酸反应又能与强碱反应的两性物质,从沉淀中分离出Cr(OH)3的流程如图:
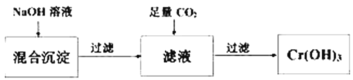
写出从该沉淀物中分离出Cr(OH)3的离子方程式__________、____________。
(4)回收所得的Cr(OH)3,经③④⑤步处理后又可转变成K2Cr2O7。纯净的K2Cr2O7常用于准确测定Na2S2O3溶液的物质的量浓度,方法如下:
①![]() +6I-+14H+=3I2+2Cr3++7H2O
+6I-+14H+=3I2+2Cr3++7H2O
②2![]() +I2=
+I2=![]() +2I-
+2I-
准确称取纯净的K2Cr2O7 0.1225g配成溶液,用上述方法滴定,消耗Na2S2O3溶液25.00mL。则Na2S2O3溶液的物质的量浓度为______(保留四位有效数字)。
【答案】①④ ![]() +6Fe2++14H+=2Cr3++6Fe3++7H2O Fe(OH)3 CaSO4 Cr(OH)3+OH-=
+6Fe2++14H+=2Cr3++6Fe3++7H2O Fe(OH)3 CaSO4 Cr(OH)3+OH-=![]() +2H2O
+2H2O ![]() +CO2+2H2O=Cr(OH)3↓+
+CO2+2H2O=Cr(OH)3↓+![]() 0.1000 mol·L-1
0.1000 mol·L-1
【解析】
(3)第①步中加入FeSO4·7H2O,Cr元素被还原,处理后的溶液中主要有Cr3+、Fe3+和SO![]() 等,加入碱石灰调节pH值,得到Cr(OH)3、Fe(OH)3和微溶于水的CaSO4;过滤后向沉淀中加入过量的NaOH溶液,Cr(OH)3溶解得到含
等,加入碱石灰调节pH值,得到Cr(OH)3、Fe(OH)3和微溶于水的CaSO4;过滤后向沉淀中加入过量的NaOH溶液,Cr(OH)3溶解得到含![]() 的溶液,过滤后向滤液中通入足量的二氧化碳得到Cr(OH)3沉淀;
的溶液,过滤后向滤液中通入足量的二氧化碳得到Cr(OH)3沉淀;
(1)上述各步反应中,第①步中![]() 转化为Cr3+,第④步中
转化为Cr3+,第④步中![]() 转化为CrO
转化为CrO![]() ,元素化合价发生变化,属于氧化还原反应,其余步骤没有元素的化合价变化,不属于氧化还原反应;
,元素化合价发生变化,属于氧化还原反应,其余步骤没有元素的化合价变化,不属于氧化还原反应;
(2)第①步中![]() 转化为Cr3+,被还原,则Fe2+被氧化为Fe3+,根据电子守恒和元素守恒可得离子方程式为
转化为Cr3+,被还原,则Fe2+被氧化为Fe3+,根据电子守恒和元素守恒可得离子方程式为![]() +6Fe2++14H+=2Cr3++6Fe3++7H2O;
+6Fe2++14H+=2Cr3++6Fe3++7H2O;
(3)沉淀物中除了Cr(OH)3外,还有Fe(OH)3以及微溶于水的CaSO4;沉淀中分离出Cr(OH)3时先用NaOH将其溶解,该过程类似氢氧化铝与NaOH溶液的反应,离子方程式为Cr(OH)3+OH-=![]() +2H2O;之后向含
+2H2O;之后向含![]() 的溶液中通入足量的CO2,得到Cr(OH)3和
的溶液中通入足量的CO2,得到Cr(OH)3和![]() ,离子方程式为
,离子方程式为![]() +CO2+2H2O=Cr(OH)3↓+
+CO2+2H2O=Cr(OH)3↓+![]() ;
;
(4)根据离子方程式①可知6n(![]() )=n(Iˉ),根据离子方程式②可知n(Na2S2O3)=n(Iˉ),所以6
)=n(Iˉ),根据离子方程式②可知n(Na2S2O3)=n(Iˉ),所以6![]() )= n(Na2S2O3),设Na2S2O3溶液的物质的量浓度为c,则0.025L×c=
)= n(Na2S2O3),设Na2S2O3溶液的物质的量浓度为c,则0.025L×c=![]() ×6,解得c=0.1000 mol·L-1。
×6,解得c=0.1000 mol·L-1。

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案【题目】无水硫酸铜(CuSO4)为白色或灰白色粉末。其水溶液显蓝色,呈弱酸性。可用作杀菌剂和电解精炼铜时的电解液。某化学课外活动小组通过设计硫酸铜受热分解的探究实验,测定气体产物的成分(已知硫酸铜完全分解,固体产物仅含CuO)。实验装置如图所示,回答下列问题

(1)实验前需进行的操作是_________,仪器a的名称是_____,加热时试管外壁必须干燥,要先均匀加热,再集中加热,其目的是__________。
(2)实验结束时根据f中量筒是否收集到水,确定气体产物中有无_______(填化学式)。
(3)装置c的作用是_________。
(4)装置d中的化学方程式为____,_____。有小组成员建议在装置d后增加连接一个干燥管,其原因是_______。
(5)按完善装置的实验结束前后测得相关数据如下:(填化学式)。
实验前无水硫酸铜的质量/g | 实验后装置d增加的质量/g | 量筒中水的体积折算成标准状况下气体的体积/mL |
6.4 | 2.56 | 224 |
通过计算,写出CuSO4受强热分解的化学方程式:______。