��Ŀ����
4��2015��8��12��23��30���ң�������������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ�軯�ƣ�����Ϊ700�����ң����ϣ��軯�ƻ�ѧʽΪNaCN����ɫ�ᾧ�������ĩ���׳��⣬�����Ŀ�������ζ���綾��Ƥ���˿ڽӴ������롢��ʳ�����ж��������۵�563.7�棬�е�1496�森������ˮ����ˮ�������軯�⣬ˮ��Һ��ǿ���ԣ���һ����Ҫ�Ļ���ԭ�ϣ����ڵ�ơ�ұ����л��ϳ�ҽҩ��ũҩ�������������森
��1�������ӷ���ʽ��ʾ��ˮ��Һ��ǿ���Ե�ԭ��CN-+H2O?HCN+OH-��
��2���軯��Ҫ��˫��ˮ������������кͣ�
����˫��ˮ��������һ����ʽ�κ�һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬��д���÷�Ӧ�Ļ�ѧ����ʽNaCN+H2O2+H2O=NaHCO3+NH3����
��������������к͵����ӷ���ʽΪCN-+S2O32-��A+SO32-��AΪSCN-���ѧʽ����
��3�������ˮ�е�CN-�о綾��
��CN-��CԪ����+2�ۣ�NԪ����-3�ۣ���ǽ�����N��C�����=��
��������������£�CN-�ܹ�������������HCO3-��ͬʱ����NH3���÷�Ӧ�����ӷ���ʽΪ2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3��
��4��K2FeO4�ܷdz���ֵ�ȥ����Ԯ�����ķ�ˮ�����¼�ƾ�Ԯ��ˮ���е�CN-���ӣ�����K2FeO4����������ֱ������з�Ӧ��
��.3CN-+2FeO42-+5H2O¾��2Fe��OH��3+3CNO-+4OH-
��.6CN-+10FeO42-+22H2O¾��10Fe��OH��3+6CO32-+3N2+14OH-
��.3CN-+8FeO42-+17H2O¾��8Fe��OH��3+3CO32-+3NO2-+10OH-
��.3CN-+10FeO42-+22H2O¾��10Fe��OH��3+3CO32-+3NO3-+14OH-
��ȡ��CN-���ӵľ�Ԯ��ˮ������һ������K2FeO4��������ҺpH=11.2����Ӧ10min��CN-���ӵ�ȥ���ʳ���99%�����ٶ�K2FeO4ֻ��CN-��Ӧ��
| ʵ�� | CN- Ũ�� | �����Ԯ��ˮ�е�K2FeO4 | ||
| ��Ԯ��ˮ | ���� | Ũ�� | ������ | |
| �� | 10mg/L | 0.082mg/L | 75mg/L | 67.14% |
��5�������£�0.1mol/L���Ȼ����Һ��0.1mol/L�����������Һ�����Ը�ǿ����NH4HSO4��
��ԭ����HSO4-�нϴ�̶ȵĵ��룬ʹ��Һ�ʽ�ǿ���ԣ���NH4Clֻ��NH4+ˮ��������ԣ���֪��H2SO4��Ki2=1.2��10-2NH3•H2O��Ki=1.8��10-5��
���� ��1���軯����ǿ�������Σ�ˮ����ʾ���ԣ�
��2����˫��ˮ�����軯�ƣ�˫��ˮ���������ԣ��軯�Ʊ��ֳ���ԭ�ԣ��õ�������һ����ʽ�μ���һ����ʹʪ��ĺ�ɫʯ����ֽ���������弴����������Ԫ���غ���Ѱ�����ʵĻ�ѧʽ��
��3��Ԫ�صĵõ�������ǿ����ʾ���ۣ�������������£�CN-�ܹ�������������HCO3-��ͬʱ����NH3�����ݷ�Ӧ��Ͳ����Ϸ�ʵ������д����ʽ��
��4�����ݲμӷ�Ӧ�����ӵ���֮�ȵõ���Ӧ���еķ���ʽ��
��5��HSO4-���ڵ���̶Ƚϴ�NH4Clֻ��NH4+����ˮ�⣮
��� �⣺��1���軯����ǿ�������Σ�ˮ����ʾ���ԣ�ˮ��ԭ��Ϊ��CN-+H2O?HCN+OH-���ʴ�Ϊ��CN-+H2O?HCN+OH-��
��2������˫��ˮ�����軯�ƣ�˫��ˮ���������ԣ��軯�Ʊ��ֳ���ԭ�ԣ��õ�������һ����ʽ�μ���һ����ʹʪ��ĺ�ɫʯ����ֽ���������弴������NaCN+H2O2+H2O=NaHCO3+NH3�����ʴ�Ϊ��NaCN+H2O2+H2O=NaHCO3+NH3����
��������������к͵����ӷ���ʽΪCN-+S2O32-��A+SO32-������Ԫ���غ㣬�õ�����A�Ļ�ѧʽSCN-���ʴ�Ϊ��SCN-��
��3����CN-��CԪ����+2�ۣ�NԪ����-3�ۣ���ǽ�����N��ǿ��C�ģ��ʴ�Ϊ������
��������������£�CN-�ܹ�������������HCO3-��ͬʱ����NH3����Ӧ�ķ���ʽΪ��2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3���ʴ�Ϊ��2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3��
��4�����ĵ�CN-Ũ����10mg/L-0.082mg/L=9.918mg/L�������Ԯ��ˮ�е�K2FeO4������Ũ�ȣ�75mg/L��67.14%=50.355mg/L�����ݷ�Ӧ��ԭ�����õ�������ӦΪ��3CN-+2FeO42-+5H2O=2Fe��OH��3+3CNO-+4OH-��CN-��FeO42-��������CNO-���ʴ�Ϊ��CNO-��
��5����ˮ��Һ�У�HSO4-�нϴ�̶ȵĵ��룬ʹ��Һ�ʽ�ǿ���ԣ����Ƕ�NH4Clֻ��NH4+ˮ��������ԣ��ʴ�Ϊ��NH4HSO4��HSO4-�нϴ�̶ȵĵ��룬ʹ��Һ�ʽ�ǿ���ԣ���NH4Clֻ��NH4+ˮ��������ԣ�
���� �����ۺ����������ԭ��Ӧ�����ӷ���ʽ����д���ε�ˮ���Լ�������ʵĵ���ȷ����֪ʶ�������ۺ�֪ʶ�Ŀ��飬�ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��������ԭ��Ӧ | B�� | ������������Ӧ | C�� | ���ǻ�ԭ�� | D�� | ��ʧȥ���� |
| A�� | XY | B�� | X3Y2 | C�� | X2Y3 | D�� | XY3 |
| A�� | ����56.0LN2����״���� | |
| B�� | ��0.300molKNO3������ | |
| C�� | ��������Nԭ�ӵ����ʵ���Ϊ4.75mol | |
| D�� | ת�Ƶ��ӵ����ʵ���Ϊ1.50mol |
| A�� | ����������ʯ��ˮ����250mL̼��������Һ�У����ˡ������õ�10g��������̼��������Һ�����ʵ���Ũ��Ϊ0.4mol•L-1 | |
| B�� | ������ά��������Ȼ��ķ���ά����ͨ����ѧ�ϳɷ����õ��� | |
| C�� | �������ﲻ�����γ����꣬���ܲ����ж��Ĺ⻯ѧ���� | |
| D�� | ̫�����Թ���ȵ���ʽ���͵����棬�ǵ��������������Դ |
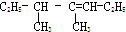 4��5-����-3-��ϩ
4��5-����-3-��ϩ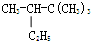 2��2��3һ��������
2��2��3һ��������