��Ŀ����
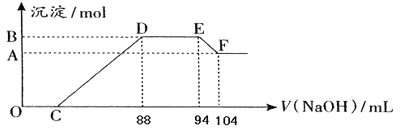
����Ŀ��N2��H2�ϳ�NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
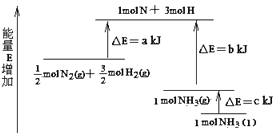
A.N2(g)��3H2(g)��2NH3(l) ��H��2(a-b-c)kJ/mol
B.N2(g)��3H2(g)��2NH3(g) ��H��2(b-a)kJ/mol
C.1/2 N2(g)��3/2H2(g)��NH3(l) ��H��(b��c-a)kJ/mol
D.1/2 N2(g)��3/2H2(g)��NH3(g) ��H��(a��b)kJ/mol
���𰸡�A
��������
����ͼ��![]() molN2(g)+
molN2(g)+![]() molH2(g)��������1molNH3(g)��������(b-a)kJ�����
molH2(g)��������1molNH3(g)��������(b-a)kJ�����![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g) ��H=(a-b)kJ/mol����1mol��NH3(g)ת��Ϊ1mol��NH3(l)�ų�������ΪckJ�������У�
H2(g)=NH3(g) ��H=(a-b)kJ/mol����1mol��NH3(g)ת��Ϊ1mol��NH3(l)�ų�������ΪckJ�������У�![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l) ��H=(a-b-c)kJ/mol������N2(g)+3H2(g)=2NH3(1) ��H=2(a-b-c)kJmol-1����ѡA��
H2(g)=NH3(l) ��H=(a-b-c)kJ/mol������N2(g)+3H2(g)=2NH3(1) ��H=2(a-b-c)kJmol-1����ѡA��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ