��Ŀ����
����Ŀ�����ҳ����ƽ���ͳ��ҵ����������������ҵ�����Ż���ҵ�ṹ�������Ŀ���ǽ��ܼ��ţ����������Ĺؼ��Ǽ���CO2�ŷţ���������������Ҫ�ֶ��Ǻ�������CO2���ش��������⣺
��1��CO2�ĵ���ʽ��___��
��2������CO2�ɺϳ�����[CO(NH2)2]���ϳ�ԭ�ϳ�CO2�⣬����NH3���÷����Ʊ����صĻ�ѧ����ʽ��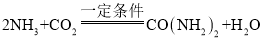 ���÷����Ʊ�����һ����
���÷����Ʊ�����һ����![]() ��2����NH3������ԭ����____��
��2����NH3������ԭ����____��
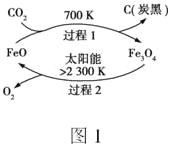
��3������̫���ܣ���CO2Ϊԭ����ȡ̿�ڵ�������ͼ1��ʾ��һ������������1������1mol̿�ڵķ�Ӧ��Ϊ��H1��������2�����Ȼ�ѧ����ʽΪ��2Fe3O4(s)![]() 6FeO(s)��O2(g) ��H2����ͼ1���Ʊ�̿�ڵ��Ȼ�ѧ����ʽΪ___��
6FeO(s)��O2(g) ��H2����ͼ1���Ʊ�̿�ڵ��Ȼ�ѧ����ʽΪ___��
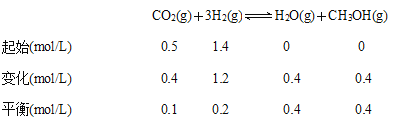
��4����1mol CO2��3mol H2�����ݻ�Ϊ1L�ĺ����ܱ������У�������Ӧ��2CO2(g)��6H2(g)![]() C2H4(g)��4H2O(g) ��H��
C2H4(g)��4H2O(g) ��H��
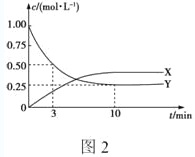
��ͼ2�Dz�õĸ÷�Ӧ��X��Y��Ũ����ʱ��仯�����ߣ�����XΪ___(д��ѧʽ)����Ӧ�ﵽƽ��ʱ��ƽ����Ӧ����v(H2)��____mol��L��1��min��1��
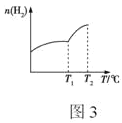
�ڲ�ͬ�¶���ƽ��ʱ�����������H2�����ʵ������¶ȵı仯������ͼ3��ʾ����÷�Ӧ����H____0(����������������������ȷ����)���ⶨ�¶�С��T2ʱ����Ӧ��ϵ����O2���ڣ���T1��T2���¶ȷ�Χ�ڣ�H2�����ʵ������������ԭ�������____��
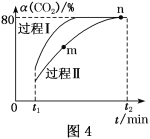
��5��CO2�����Ժϳɼ״���CO2(g)��3H2(g)![]() H2O(g)��CH3OH(g) ��H����53.7 kJ��mol��1��һ�������£���1 mol CO2��2.8 mol H2�����ݻ�Ϊ2L�ľ����ܱ������У�����������Ӧ��CO2��ת����[��(CO2)]�ڲ�ͬ������������ʱ��ı仯������ͼ4��ʾ��n���ƽ�ⳣ��K��___��
H2O(g)��CH3OH(g) ��H����53.7 kJ��mol��1��һ�������£���1 mol CO2��2.8 mol H2�����ݻ�Ϊ2L�ľ����ܱ������У�����������Ӧ��CO2��ת����[��(CO2)]�ڲ�ͬ������������ʱ��ı仯������ͼ4��ʾ��n���ƽ�ⳣ��K��___��
���𰸡�![]() NH3��Һ����������ˮ������β������
NH3��Һ����������ˮ������β������ 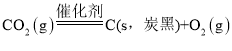 ��H����H1����H2 C2H4 0.225 �� ��ϩ�ֽ�����H2 200
��H����H1����H2 C2H4 0.225 �� ��ϩ�ֽ�����H2 200
��������
��1��CO2�ĵ���ʽ��![]() ��
��
��2���ڷ�Ӧ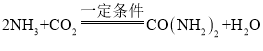 �У�ʹ�ù�����NH3������ʹCO2��Ӧ����ȫ����������NH3������Һ��������ˮ�ķ������ա��ʴ�Ϊ��NH3��Һ����������ˮ������β�����գ�
�У�ʹ�ù�����NH3������ʹCO2��Ӧ����ȫ����������NH3������Һ��������ˮ�ķ������ա��ʴ�Ϊ��NH3��Һ����������ˮ������β�����գ�
��3������ͼ�У�������1�����Ȼ�ѧ����ʽΪ��CO2(g)+6FeO(s) ![]() C(s��̿��)+2Fe3O4(s) ��H1���� ������2�����Ȼ�ѧ����ʽ��Ӽ��ɵõ��ܷ�Ӧ���Ȼ�ѧ����ʽ��
C(s��̿��)+2Fe3O4(s) ��H1���� ������2�����Ȼ�ѧ����ʽ��Ӽ��ɵõ��ܷ�Ӧ���Ȼ�ѧ����ʽ��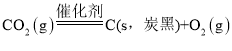 ��H����H1����H2��
��H����H1����H2��
��4���ٴ�ͼ2���Կ�����X��������ӿ�ʼ��Ӧ��ƽ�⣬Ũ�ȱ仯��0.375mol/L��Y�Ƿ�Ӧ��ӷ�Ӧ��ʼ��ƽ�⣬Ũ�ȱ仯��1.00-0.25=0.75mol/L��X��Y��Ũ�ȱ仯֮��Ϊ1:2�������ʵ�Ũ�ȱ仯֮�ȵ��ڷ���ʽ�Ļ�ѧ������֮�ȣ����ݻ�ѧ����ʽ��֪X��Y�ֱ���C2H4��CO2����Ӧ�ﵽƽ��ʱ��ƽ����Ӧ����v(CO2)��0.75mol/L��10min=0.075mol/(L��min)����һ����Ӧ�У��ò�ͬ���ʱ�ʾ������֮�ȵ��ڻ�ѧ������֮�ȣ�������H2��ʾ�Ļ�ѧ��Ӧ����Ϊ0.075mol/(L��min)��3=0.225 mol/(L��min)���ʴ�ΪC2H4��0.225��
��ͼ3�У������¶ȣ�H2�����ʵ�������˵�������¶ȣ�ƽ�������ƶ������Ը÷�Ӧ����H��0���¶�С��T2ʱ����Ӧ��ϵ����O2���ڣ�˵��H2Oδ�ֽ⣬��T1��T2���¶ȷ�Χ�ڣ�H2�����ʵ������������ԭ���������ϩ�ֽ�����H2��
��5��ͼ4�У���n�㣬������̼��ת����Ϊ80%��Ӧ��������ʽ�������㣺
��n���ƽ�ⳣ��K��![]() ��
��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�����Ŀ�����мס���������ѧС������������ͬװ�ã�ͨ���ⶨ������ͬ�����������ʱ�䳤����̽��Ӱ��H2O2�ֽ����ʵ�����(��һ�������ı�)����С��������ʵ����Ʒ�����
ʵ���� | �¶� | ���� | Ũ�� |
����ʵ��� | 25�� | ���������� | 10mL 5%H2O2 |
����ʵ��� | 25�� | �������� | 10mL 5%H2O2 |
�ס�����С��ó���ͼ���ݣ�
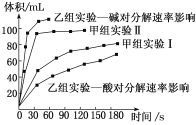
���ڸ�ʵ��������������ȷ����
A. 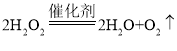 ��O2����������
��O2����������
B. ��С��ʵ��ó��Ľ�����Fe2O3��MnO2��Ч�ʸ���
C. ��С��ʵ��Ҳ���Բⶨ25��ʱ������ͬʱ���ڲ������������
D. �����о���Ӱ�����ص����ݷ�������ͬ������H2O2�ڼ��Ի����·ų��������ʽϿ�