题目内容
14.海水是宝贵的自然资源,将海水淡化与浓海水资源化结合起来是综合利用海水的重要途径之一.(1)采用“空气吹出法”从浓海水吹出Br2,并用纯碱吸收,这样做的目的是使Br2富集;碱吸收溴的主要反应是Br2+Na2CO3+H2O→NaBr+NaBrO3+NaHCO3,吸收0.15mol Br2时,转移的电子为0.25mol.
(2)海水提镁的一段工艺流程如图:
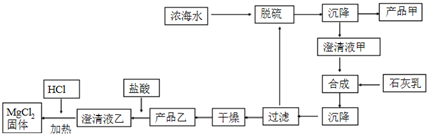
浓海水的主要成分如下:
| 离子 | Na+ | Mg2+ | Cl- | SO42- |
| 浓度/(g•L-1) | 63.7 | 28.8 | 144.6 | 46.4 |
②产品乙的化学式为Mg(OH)2,1L浓海水最多可得到产品乙的质量是69.9g.
分析 (1)吹出Br2,用SO2吸收,发生氧化还原反应生成硫酸和HBr,Br2+Na2CO3+H2O→NaBr+NaBrO3+NaHCO3中Br由0降低为-1,由0升高为+5价,可知吸收3mol溴转移5mol电子;
(2)由流程可知,浓海水中利用钙离子将硫酸根离子转化为沉淀,得到产品甲为硫酸钙;滤液甲在合成步骤中加入石灰乳,将镁离子转化为沉淀,过滤、干燥后得到的产品乙为氢氧化镁沉淀;计算1L溶液中Mg2+的质量,根据Mg2+~Mg(OH)2计算氢氧化镁的质量.
解答 解:(1)吹出Br2,用SO2吸收,发生氧化还原反应生成硫酸和HBr,其目的是使Br2富集;Br2+Na2CO3+H2O→NaBr+NaBrO3+NaHCO3中Br由0降低为-1,由0升高为+5价,可知吸收3mol溴转移5mol电子,则吸收0.15mol Br2时,转移的电子为0.15mol×5353=0.25mol,
故答案为:使Br2富集;0.25;
(2)由流程可知,浓海水中利用钙离子将硫酸根离子转化为沉淀,得到产品甲为硫酸钙;滤液甲在合成步骤中加入石灰乳,将镁离子转化为沉淀,过滤、干燥后得到的产品乙为氢氧化镁沉淀
①该工艺过程中,脱硫阶段主要的离子方程式为Ca2++SO42-=CaSO4↓,加入石灰乳时所发生的离子方程式是Mg2++Ca(OH)2=Mg(OH)2↓+Ca2+,
故答案为:Ca2++SO42-=CaSO4↓;Mg2++Ca(OH)2=Mg(OH)2↓+Ca2+;
②由上述分析可知,产品乙为Mg(OH)2,
溶液中m(Mg2+)=1L×28.8g/L=28.8g,
Mg2+~Mg(OH)2
24g 58g
28.8g m[Mg(OH)2]
m[Mg(OH)2]=28.8g×58g24g58g24g=69.6g,
故答案为:Mg(OH)2;69.9g.
点评 本题考查海水资源开发利用,为高频考点,涉及氧化还原反应、离子反应及流程分析,注重基础知识的综合运用,侧重分析与应用能力的考查,题目难度中等.

 阅读快车系列答案
阅读快车系列答案| A. | 溴苯中混有溴,加入KI溶液,振荡,用汽油萃取出溴 | |
| B. | 乙烷中混有乙烯,通H2在一定条件下反应,使乙烯转化为乙烷 | |
| C. | 硝基苯中混有浓H2SO4和浓HNO3,将其倒入NaOH溶液中,静置,分液 | |
| D. | 乙烯中混有CO2和SO2,将其通过盛有NaOH溶液的洗气瓶 |
| A. | 淀粉(氯化钠)渗析 | |
| B. | 硬脂酸钠(甘油溶液)盐析、过滤 | |
| C. | 水(鸡蛋清)蒸馏 | |
| D. | 蔗糖(葡萄糖)与银氨溶液混合水浴加热,过滤 |
| A. | 2NaCl(熔融)电解_电解–––––2Na+Cl2↑ | B. | MgO+H2△_△––––––Mg+H2O | ||
| C. | Fe2O3+3CO高温_高温–––––2Fe+3CO2 | D. | 2Ag2O△_△––––––4Ag+O2↑ |
| A. | “水滴石穿”主要是溶解了CO2的雨水与CaCO3长期作用生成了可溶性Ca(HCO3)2的缘故 | |
| B. | 长期盛放NaOH溶液的试剂瓶不易打开,是因为NaOH与瓶中的CO2反应导致瓶内气体减少形成负压的缘故 | |
| C. | 严格地讲,实验室使用”通风橱”防污染是不负责任的,因为实验产生的有害气体没有得到转化或吸收 | |
| D. | “雨后彩虹”与“海市蜃楼”都是自然界的光学现象,也与胶体知识有关 |
| A. | 酸性:H4SiO4<H3PO4<H2SO4<HClO4 | B. | 碱性:Ca(OH)2>Mg(OH)2>Al(OH)3 | ||
| C. | 氢化物的稳定性:SiH4>H2S>H2O>HF | D. | 原子半径:F<O<S<Na |
| A. | 放电时,LiMn2O4发生氧化反应,电池内部Li+向正极移动 | |
| B. | 放电时,正极反应为:Li++LiMn2O4+e-═Li2Mn2O4 | |
| C. | 醋酸可用作锂离子电池的电解质 | |
| D. | 充电时,锂的碳材料为阳极且反应为:Li++e-═Li |
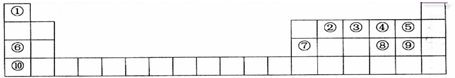
 ,其最高氧化物水化物与⑥号元素最高价氧化物水化物反应的离子方程式为Al(OH)3+OH-=[Al(OH)4]-_.
,其最高氧化物水化物与⑥号元素最高价氧化物水化物反应的离子方程式为Al(OH)3+OH-=[Al(OH)4]-_.