��Ŀ����
19���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ�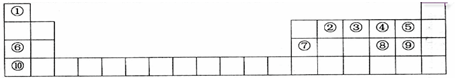
��1����������õĽ���������õķǽ����γɵĻ�ѧʽ��KF���������ӣ�
��2���ߺ�Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
 �������������ˮ�������Ԫ�����������ˮ���ﷴӦ�����ӷ���ʽΪAl��OH��3+OH-=[Al��OH��4]-_��
�������������ˮ�������Ԫ�����������ˮ���ﷴӦ�����ӷ���ʽΪAl��OH��3+OH-=[Al��OH��4]-_����3���ߺ�Ԫ�ض�Ӧ�ĵ��ʼ������ᷴӦ��Ҳ����Ӧ��д�����Ԫ�����������ˮ���ﷴӦ�Ļ�ѧ����ʽΪ2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2��
��4���ڡ��ۡ������ۺ������������ǿ������˳����HClO4��HNO3��H2CO3��д��ѧʽ�����ܡ��ݡ����⻯���ȶ�����ǿ������˳����HF��H2O��H2S��д��ѧʽ����
��5���١��ۡ�������Ԫ���γɵĻ������л�ѧ�������ͣ����Ӽ����ۼ����仯��������Ϊ���ӻ����
��6���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2=2Na2CO3+O2��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪�١���ֱ�ΪH��C��N��O��F��Na��Al��S��Cl��K��
��1������õĽ���ΪK��������õķǽ���F�γɵĻ�����ΪKF��
��2����������Ϊ���������������Ӧ����[Al��OH��4]-��
��3����������������Һ��Ӧ����Na[Al��OH��4]��������
��4��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���
��5���١��ۡ�������Ԫ���γɵĻ�����ΪNH4F��Ϊ���ӻ����
��6��CO2��Na2O2����������ԭ��Ӧ����̼���ƺ�������
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪�١���ֱ�ΪH��C��N��O��F��Na��Al��S��Cl��K��
��1������õĽ���ΪK��������õķǽ���F�γɵĻ�����ΪKF��Ϊ���ӻ�����������Ӽ����ʴ�Ϊ��KF�����ӣ�
��2����ΪAl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3��ԭ�ӽṹʾ��ͼΪ ����������Ϊ���������������Ӧ����[Al��OH��4]-������ʽΪAl��OH��3+OH-=[Al��OH��4]-��
����������Ϊ���������������Ӧ����[Al��OH��4]-������ʽΪAl��OH��3+OH-=[Al��OH��4]-��
�ʴ�Ϊ�� ��Al��OH��3+OH-=[Al��OH��4]-��
��Al��OH��3+OH-=[Al��OH��4]-��
��3����������������Һ��Ӧ����Na[Al��OH��4]����������Ӧ�ķ���ʽΪ2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2����
�ʴ�Ϊ��2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2����
��4���ǽ�����Cl��N��C��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ��ΪHClO4��HNO3��H2CO3��
�ǽ�����F��O��S��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���ΪHF��H2O��H2S��
�ʴ�Ϊ��HClO4��HNO3��H2CO3�� HF��H2O��H2S��
��5���١��ۡ�������Ԫ���γɵĻ�����ΪNH4F��Ϊ���ӻ�����������Ӽ����ۼ����ʴ�Ϊ�����Ӽ����ۼ��� ���ӻ����
��6���ں͢��γɵĻ�����ΪCO2���ܺ͢��γɵĻ�����ΪNa2O2�����߷���������ԭ��Ӧ����̼���ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
�ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
���� ���⿼��Ԫ�������ɺ����ڱ����ۺ�Ӧ�ã���Ŀ�ѶȲ���ע�����Ԫ�������ڱ��е�λ��ȷ��ԭ�ӽṹ������Ԫ�������ɵĵݱ���ɣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
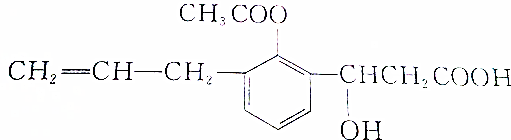
Сѧ��10����Ӧ����ϵ�д�| A�� | ��Ũ��������ֵ�������Һ�͵�����Һ | |
| B�� | �ڵ�������Һ�еμ�����ͭ��Һ�����ɰ�ɫ��������ˮ�����ܽ� | |
| C�� | ����ʵ����ڼ��뱥��ʳ��ˮʹ��֬���������������ڻ��Һ���棬ͨ��ɴ������ȥˮ�� | |
| D�� | ȡ����ˮ��Һ����������Cu��OH��2��Һ�����ȣ�δ��ש��ɫ�������ɣ�˵��������δˮ�� |
��1�����á���������������Ũ��ˮ����Br2�����ô������գ���������Ŀ����ʹBr2�����������������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������0.15mol Br2ʱ��ת�Ƶĵ���Ϊ0.25mol��
��2����ˮ��þ��һ�ι���������ͼ��
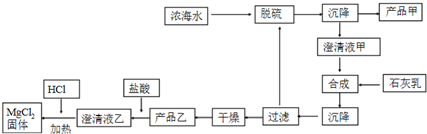
Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�ڲ�Ʒ�ҵĻ�ѧʽΪMg��OH��2��1LŨ��ˮ���ɵõ���Ʒ�ҵ�������69.9g��
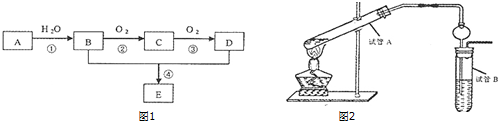
 �л���A��B��C��D������ת����ϵ���ش��������⣺
�л���A��B��C��D������ת����ϵ���ش��������⣺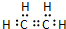 �������к��еĹ��ۼ������зǼ��Լ������Լ�����Լ���Ǽ��Լ�����
�������к��еĹ��ۼ������зǼ��Լ������Լ�����Լ���Ǽ��Լ�����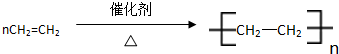 ����Ӧ���ͣ��Ӿ۷�Ӧ��
����Ӧ���ͣ��Ӿ۷�Ӧ��