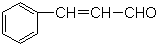��Ŀ����
19��������㷺��Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�������üױ���������ȡ�����ᣮ��Ӧԭ����
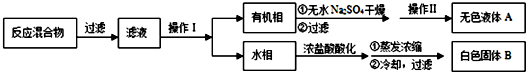
ʵ�鷽����һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ������ͼ���̷����������ͻ���δ��Ӧ�ļױ���
��֪����������Է�������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����������л���һ�㶼�й̶��۵㣮
��1��������Ϊ��Һ��������Ϊ����
��2����ɫҺ��A�Ǽױ������Լ���A���Լ�������KMnO4��Һ��
��3���������ؽᾧ��ʵ��������裺�����ܽ⡢���ȹ��ˡ�����ϴ�ӡ����װƿ����Һ��ȴ�����е�ʵ�������о��������������ȹ��ˡ���ԭ���ǣ����ٹ���ʱ���������ʧ��
���� һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ������ͼ���̷����������ͻ���δ��Ӧ�ļױ���������������ˮ���ױ�������ˮ���������ܵ�Һ����÷�Һ�������룬����ʵ��Ŀ��֪���Ӷ��õ��л����ˮ�࣬�л����к��мױ���ˮ���к��б����ᣬ�л����еļױ����������õ���ɫҺ��A��A�Ǽױ�����ˮ�������ữ������Ũ�������ݱ�������ܽ��֪���õ��Ĺ���B�DZ����ᣬ
��1�����뻥�����ܵ�Һ����÷�Һ���������뻥���ҷе㲻ͬ��Һ�����������
��2���ױ���ʹ���Ը��������Һ��ɫ��
��3����Һ�¶ȸ�ʱ��������ܽ�ȴ����¶Ȼ����������ᾧ�壮
��� �⣺һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ������ͼ���̷����������ͻ���δ��Ӧ�ļױ���������������ˮ���ױ�������ˮ���������ܵ�Һ����÷�Һ�������룬����ʵ��Ŀ��֪���Ӷ��õ��л����ˮ�࣬�л����к��мױ���ˮ���к��б����ᣬ�л����еļױ����������õ���ɫҺ��A��A�Ǽױ�����ˮ�������ữ������Ũ�������ݱ�������ܽ��֪���õ��Ĺ���B�DZ����ᣬ
��1�����뻥�����ܵ�Һ����÷�Һ��������������ͼ�У�ˮ����л�����ܣ����Բ��÷�Һ�������룬������IΪ��Һ���л��������ʻ����ҷе㲻ͬ�����Կ��Բ����������룬������IIΪ����
�ʴ�Ϊ����Һ������
��2��ͨ�����Ϸ���֪��A�Ǽױ����ױ����м��������ܱ����Ը����������Ϊ�������ʹ���Ը��������Һ��ɫ������������Ը��������Һ����ױ���
�ʴ�Ϊ���ױ�������KMnO4��Һ��
��3����Һ�¶ȸ�ʱ��������ܽ�ȴ����¶Ȼ����������ᾧ�壬���Թ���ʱҪ���ȹ��ˣ��Է��¶Ƚ��ͣ�ʹ�����������������ʧ�����Գ��ȹ��˵�Ŀ���Ǽ��ٹ���ʱ���������ʧ��
�ʴ�Ϊ���о������������ٹ���ʱ���������ʧ��
���� ���⿼�������ʵ��Ʊ��Լ������ķ�����ᴿ�����ؿ���ѧ���ķ�������������ʵ�������������ȷ���ʵ������ǽⱾ��ؼ���֪���������ʵ�����ѡȡ���ʵķ��뷽������Ŀ�Ѷ��еȣ�

| A�� | CO2��NO2 | B�� | B3H6N3��C6H6 | C�� | CH4��NH4+ | D�� | H2O��CH4 |
| A�� | 40.7 | B�� | 46 | C�� | 36 | D�� | 44 |
| A�� | �ڢۢ� | B�� | �ۢܢ� | C�� | �ڢܢ� | D�� | �ܢ� |
| A�� | ����������Ӧˮ��������ԣ�H2SiO3��H3PO4��H2SO4 | |
| B�� | ����ˮ�ķ�Ӧ���ʣ�K��Na��Mg | |
| C�� | ���ȶ��ԣ�HF��HCl��H2S | |
| D�� | ���Ӱ뾶��Cl-��F-��Na+ |
| A�� | ���ԣ�NaOH��Mg��OH��2��Al��OH��3 | B�� | ԭ�Ӱ뾶��Na��Mg��Al | ||
| C�� | ���ӵ������ԣ�Na+��Mg2+��Al3+ | D�� | ���ʵĻ�ԭ�ԣ�Na��Mg��Al |
| A�� | $\overline{V}$��NH3��=0.010 mol•L-1•s-1 | B�� | $\overline{V}$��O2��=0.001 0 mol•L-1•s-1 | ||
| C�� | $\overline{V}$��NO��=0.001 0 mol•L-1•s-1 | D�� | $\overline{V}$��H2O��=0.045 mol•L-1•s-1 |
| Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| x ֵ | 0.98 | 1.57 | 2.04 | 2.55 | 3.44 | 3.98 | 0.93 | 1.61 | 1.90 | 2.19 | 2.58 | 3.16 |
2.55��x ��N����3.44��0.93��x ��Mg����1.57��
��2��ij�л�������ṹ�к�S-N�����乲�õ��Ӷ�ƫ��дԭ�����ƣ���
��3��������ɸ������ǣ����ɼ�����ԭ����ӦԪ�ص� x ��ֵ��x��1.7ʱ��һ��Ϊ���Ӽ�������x��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3�л�ѧ�������ǹ��ۼ���
��4��Ԥ�����ڱ��У�xֵ��С��Ԫ��λ��������IA�壮��������Ԫ�س��⣩