��Ŀ����
2��ij������ȤС���H2O2�ķֽ�������������ʵ��̽������1���±��Ǹ�С���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ���l0mL H2O2��ȡ150mL O2�����ʱ�ʣ�s��
| Ũ�� ʱ�䣨s�� ��Ӧ���� | 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 |
| �����������ȣ�t1�棩 | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������ȣ�t2�棩 | 360��a�� | 480 | 540 | 720 |
| MnO2���������ȣ�t2�棩 | 10��b�� | 25 | 60 | 120 |
�ڶ��շ�Ӧ����a��b������˵������ ���ضԹ�������ֽ��������Ӱ�죬��Ӱ��ɾ������Ϊ�����ӿ�ֽ����ʣ�
��2����������ͬ���ۼ�״̬��ͬ��MnO2�ֱ���뵽5mL 5%��˫��ˮ�У����ô����ǵ�ľ�����ԣ����Խ�����£�
| ������MnO2�� | ������� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ��ϲ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3min |
| ��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
������ʵ���У�ʹ�÷�ĩ״�����Ϳ�״�������ù�������ֽⷴӦ�ķ�Ӧ����֮��Ϊ10��1��
���� ��1������������Һ��ʵ������ȡ��������ҪҩƷ���ڳ����º��ѷֽ�õ���������ֽ��ٶ���Ũ�ȡ��¶ȡ����������ص�Ӱ�����ʵ�鷽����֤��ʱ��Ҫע��ʵ��Ŀ��Ʊ�������ȷ��ʵ������ȷ�ԣ�
��2�����������ǹ�������ֽ�Ĵ������ɴ�����ľ����ȼ����֪����ɴ˿�д������ʽ����ʵ������ʱ���֪���Ӵ������ͬ����Ӧ���ʲ�ͬ��ʱ�������ʳɷ��ȣ�
��� �⣺��1�����ɱ��и��������ݣ����������ȵ�����£���ͬŨ�ȵĹ���������Һ���Ǽ�������Ӧ�����������ȵ�����£���ͬŨ�ȵĹ���������Һ���ֽ⣬˵����������ķֽ��������¶��йأ����ǵõ���ͬ�����ʱ�䲻ͬ��Ũ��Խ��Ӧ���ٶ�Խ�죬˵����������ķֽ�������Ũ���йأ��Ƚ�ͬһŨ�ȵĹ���������Һ��30%ʱ�����������ȵ�ʱ����Ҫʱ����360s���д������ȵ������£���Ҫʱ����10s��˵����������ķֽ��������¶ȡ������йأ�
�ʴ�Ϊ���¶ȡ�Ũ�ȡ�������
�ڶ��շ�Ӧ����a��b��Ũ�ȡ��¶���ͬ����Ӵ����ķ�Ӧ���ʿ죬�ʴ�Ϊ�������������ӿ�ֽ����ʣ�
��2����H2O2�ڶ������������·�����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
����������������ͬʱ����ĩ״�������̱ȿ�״�������̷�Ӧ����ʱ��̣�˵���Ӵ�����Է�Ӧ������Ӱ�죬ʹ�÷�ĩ״�����Ϳ�״�������ù�������ֽⷴӦ�ķ�Ӧ����֮��Ϊ30min��3min=10��1���ʴ�Ϊ��10��1��
���� ���⿼�鷴Ӧ���ʵ�ʵ��̽����Ϊ��Ƶ���㣬���ձ��������ݡ���Ӧ���ʵ�Ӱ�����ء����Ʊ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | ��ϵ��ɫ��ԭ���� | B�� | ��ϵ��ɫ��ԭ��dz | ||
| C�� | ��ϵ��ɫ���� | D�� | ע������ѹǿ���� |
| A�� | Ca2+��Al3+��NO3-��Cl- | B�� | H+��Fe2+��SO42-��NO3- | ||
| C�� | K+��NH4+��CO32-��OH-�� | D�� | Na+��Mg2+��CO32-��Br- |
| ��1����ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45���� | ��1���л���A����Է�������Ϊ��90�� |
| ��2������9.0gA��������O2���ȼ�գ���ʹ ���������ͨ��������ˮ����ͭ��ĩ������ʯ��ˮ����������ͭ��ĩ����5.4g��ʯ��ˮ����30.0g��ɫ�������ɣ� | ��2��9.0g�л���A��ȫȼ��ʱ�������㣺 ����CO2��Ϊ0.3mol�� ���ɵ�H2O0.3mol�� �л���A�ķ���ʽC3H6O3�� |
| ��3����������ײⶨ��֤ʵ���к���O-H����-COOH���ţ�C-H�������˴Ź������IJⶨ����˴Ź�������ͼ �磺  | ��3��A�Ľṹ��ʽ �� �� |
| ��4�������������ײⶨ��A��һ��ͬ���칹���У�����O-H���� ������ȩ����C-O����  �˴Ź������IJⶨ�� ��˴Ź�������ͼ���ң� | ��4��A������ͬ���칹��Ľṹ��ʽΪ�� CH2��OH��CH��OH��CHO�� |
| ��5�������������ײⶨ��A��һ��ͬ���칹���У�����O-H����������C=O��C-O���� �˴Ź������IJⶨ�� ��˴Ź�������ͼ�磺  | ��5��A������ͬ���칹��Ľṹ��ʽΪ�� �� �� |
| A�� |  ���Ȼ�狀�����������NH3 | B�� |  �������̺�Ũ���������� | ||
| C�� |  ��п����ϡ������H2 | D�� |  ��˫��ˮ�Ͷ���������O2 |
| A�� | ��ֱ��������������̼ԭ�Ӳ���һ��ֱ���� | |
| B�� | �ڹ����������ܹ�����������ȡ����Ӧ | |
| C�� | �������ͬ���칹�嶡������������������ | |
| D�� | ȼ��ʱ��Ҫ�ǽ���ѧ��ת��Ϊ���ܺ��� |
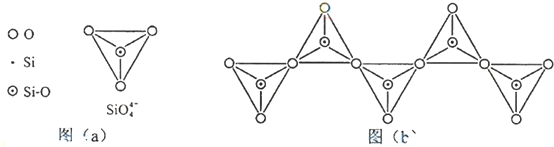
| A�� | ��������̼�ĵ���ʽΪ | B�� | C3O2��CO��CO2����̼�������� | ||
| C�� | C3O2��COһ������ȼ������CO2 | D�� | C3O2��CO2����̼������� |
 ��
��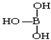 ������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H+����д����������ˮ����Һ�����Ե����ӷ���ʽ��H3BO3+H2O?H4BO4-+H+��
������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H+����д����������ˮ����Һ�����Ե����ӷ���ʽ��H3BO3+H2O?H4BO4-+H+��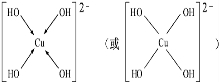 ��
��