��Ŀ����
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�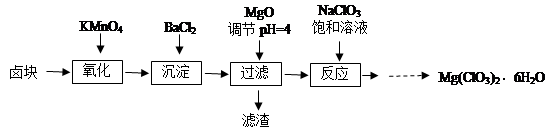
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S )���¶�(T )�仯������ͼ��ʾ��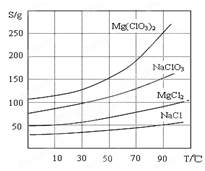
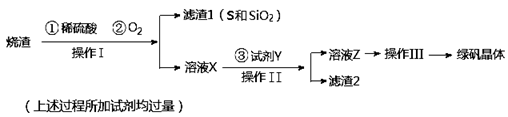
��1����������Ҫ����Ҫ���������� ��
��2������BaCl2��Ŀ���� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ�ʣ���Fe2�����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ�� ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
��1��©�������������ձ�
��2����ȥSO42�� BaSO4��Fe(OH)3
��3��MgCl2��2NaClO3��Mg(ClO3)2��2NaCl����δд�������Ų����֣�
��4����ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O ��78.3%
���������������1����������Ҫ����Ҫ����������©�������������ձ�����2������BaCl2��Ŀ����ʹ����SO42-ת��Ϊ������ȥ����MgO������Һ��PH��4����ʱFe3+���γ�Fe(OH)3����.��ͬ����BaCl2����������ᱵ����һ����˳�ȥ�����Թ���������������Ҫ�ɷ�ΪBaSO4��Fe(OH)3����3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪMgCl2��2NaClO3��Mg(ClO3)2��2NaCl������Mg(ClO3)2����Һ�еõ�Mg(ClO3)2��6H2O���������ܽ�Ƚϴ����¶ȵ�Ӱ��仯�ϴ���ص��������ᾧ���ٳ��ȹ��ˣ������ȴ�ᾧ�õ�����4������������ԭ��Ӧ���������õ��ĵ����뻹ԭ��ʧȥ�ĵ�������������м��㡣��д������2�з�����Ӧ�����ӷ���ʽ��ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O������10.00 mL��Һ��1��0.02=3��2��0.015��0.1+ 6��n(ClO3�� ).���n(ClO3�� )="(" 0.011/6)mol.����3.50 g��Ʒ�к��е�Mg(ClO3)2��6H2O����Ϊ{( 0.011/6)mol��2}��10��299g/mol=2.741g.���Բ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ(2.741g��3.50 g)��100��=78.3%
���㣺���鳣��������������ݼ�������þ[Mg(ClO3)2]����ȡ���ɷֲⶨ��֪ʶ��

�������ױ���ҽҩ��Ⱦ�ϵȹ�ҵ��һ����Ҫ�л��м��壬������Ũ����Ϊ��������Ũ����Ϊ������ͨ���ױ���������Ӧ�Ʊ���
һ���µ��Ʊ��������ױ���ʵ�鷽���ǣ��Է�������Ϊ������������NaHSO4Ϊ����(��ѭ��ʹ��)����CCl4��Һ�У�����������(����ˮ����)��45 �淴Ӧ1 h����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ���
(1)����ʵ���й��˵�Ŀ����_____________________________________��
(2)��Һ�ڷ�Һ©����ϴ�Ӿ��ú��л��㴦��________��(��ϡ����¡�)����Һʱ��������Һ�����������������ԭ�����Һ©�����������⣬����_________________________________________________________��
(3)���и����˴������༰�����Լױ�������ӦӰ���ʵ������
| ���� |  | ���������и����칹����������(%) | �ܲ���(%) | ||
| �������ױ� | �������ױ� | �������ױ� | |||
| ŨH2SO4 | 1.0 | 35.6 | 60.2 | 4.2 | 98.0 |
| 1.2 | 36.5 | 59.5 | 4.0 | 99.8 | |
| NaHSO4 | 0.15 | 44.6 | 55.1 | 0.3 | 98.9 |
| 0.25 | 46.3 | 52.8 | 0.9 | 99.9 | |
| 0.32 | 47.9 | 51.8 | 0.3 | 99.9 | |
| 0.36 | 45.2 | 54.2 | 0.6 | 99.9 | |
��NaHSO4���Ʊ��������ױ�ʱ��������ױ���������ʵ���֮��Ϊ________��
���ɼױ������õ��ĸ��ֲ���ĺ�����֪���ױ�������Ӧ���ص���_________________________________________________________��
����Ũ������ױ�������ȣ�NaHSO4���ױ��������ŵ���________________��________________��
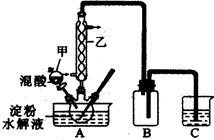
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ����ʡ�ijС����ʵ�������ý�������ȡ���Ʊ�����ͭ��
(1)������Ϊ________���������õ��IJ����������ձ���________��
(2)�������������ҪĿ����________������ͭԪ�ء�
(3)С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2(OH)2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu(OH)2��Cu4(OH)6SO4��
��֪Cu(OH)2��Cu2(OH)2CO3��Cu4(OH)6SO4��������ˮ����������ֽ��¶�����Ϊ80 �桢200 �桢300 �档
���ʵ���������Һ�ɷ֣���ɱ������ݡ�
��ѡ�Լ���2 mol��L��1���ᡢ1 mol��L��1 H2SO4��0.1 mol��L��1 NaOH��Һ��0.1 mol��L��1 BaCl2��Һ������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У�______________________ | ____________��˵������Һ�л���Cu4(OH)6SO4 |
| ����2����ȡ��������Һ���Թ��У�____________________ | ____________��˵������Һ�л���Cu(OH)2 |
(4)����ʵ����Ҫ100 mL 0.5 mol��L��1��CuSO4��Һ������ʱ���ȡ________g CuSO4��5H2O(��Է���������250)��
��ͼ��ʵ�����Ʊ�1��2���������鲢����һϵ�����ʵ���װ�ã����ȼ��г��豸���ԣ���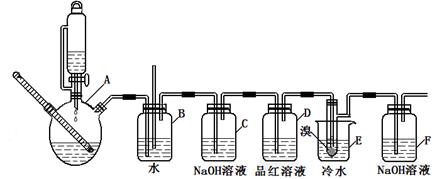
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g/cm3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | һl30 | 9 | -1l6 |
��1��A��ҩƷΪ1:3����ˮ�Ҵ���Ũ������Һ��д���Ʊ���ϩ�Ļ�ѧ��Ӧ����ʽ��____________________________________________________________��
��2�����巢��װ��ʹ����ͨ��Һ©����ԭ��_________________________________________��
��3��װ��D��Ʒ����Һ��������_______________��ͬʱBװ���ǰ�ȫƿ�����ʵ�����ʱE���Ƿ�����������д������ʱ������_______________________________________��
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ����___________________________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_____________________________________________��
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ�����__________________�����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ����_______________________________________��
��6����ѧ�������װ��F�пɸ������������Ȼ�̼Һ�����ն�������壬�жϸ������Ȼ�̼Һ���Ƿ����______����ǡ�������ԭ����____________________________��
 3H2C2O4��9NO2����3NO����9H2O
3H2C2O4��9NO2����3NO����9H2O

 H+ + OH����HCO3��
H+ + OH����HCO3��