��Ŀ����
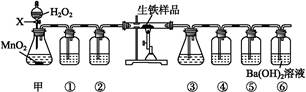
��ͼ��ʵ�����Ʊ�1��2���������鲢����һϵ�����ʵ���װ�ã����ȼ��г��豸���ԣ���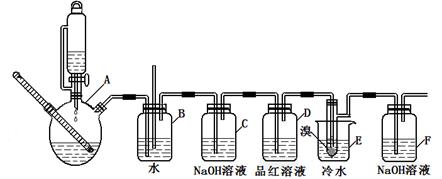
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g/cm3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | һl30 | 9 | -1l6 |
��1��A��ҩƷΪ1:3����ˮ�Ҵ���Ũ������Һ��д���Ʊ���ϩ�Ļ�ѧ��Ӧ����ʽ��____________________________________________________________��
��2�����巢��װ��ʹ����ͨ��Һ©����ԭ��_________________________________________��
��3��װ��D��Ʒ����Һ��������_______________��ͬʱBװ���ǰ�ȫƿ�����ʵ�����ʱE���Ƿ�����������д������ʱ������_______________________________________��
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ����___________________________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_____________________________________________��
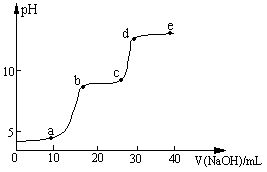
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ�����__________________�����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ����_______________________________________��
��6����ѧ�������װ��F�пɸ������������Ȼ�̼Һ�����ն�������壬�жϸ������Ȼ�̼Һ���Ƿ����______����ǡ�������ԭ����____________________________��
��1��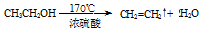 ��2�֣�
��2�֣�
��2��ʹ��ͨ��Һ©�������Һ�����£�2�֣�
��3����֤���������Ƿ���;��������Һ��������2�֣���
��4������Һ��ӷ�;1,2����������������ɹ��������������2�֣�
��5��E��Һ���ɺ���ɫ��Ϊ��ɫ;����Ӧ������Ӧ̫���ң�2�֣���
��6����;��ϩ��Һ������������Ȼ�̼��2�֣�
���������������1���ڼ��Ⱥʹ������������Ҵ�������ȥ��Ӧ������ϩ��Ȼ����ϩ����ˮ�����ӳɷ�Ӧ����1��2���������飬��Ӧ�Ļ�ѧ����ʽ��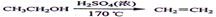 ��
��
��2�����巢��װ��ʹ����ͨ��Һ©������Ҫ����Һ�����µĴ���ѹǿ��ͬ��ʹ��ͨ��Һ©�������Һ�����¡�
��3��װ��D��Ʒ����Һ����������֤���������Ƿ���;��ͬʱBװ���ǰ�ȫƿ����E�з�����������������Һ��϶���������
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ���Ǽ���Һ��ӷ�;�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����1,2����������������ɹ��������������
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ�����E��Һ���ɺ���ɫ��Ϊ��ɫ�����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ���Ǹ���Ӧ�������������ѻ�Ӧ̫����ʹ�����Ҵ��ӷ���
��6����ϩ��Һ������������Ȼ�̼���ɽ��г�����գ��ʿ��С�
���㣺����1��2�����������Ʊ����й�ʵ��̽����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��
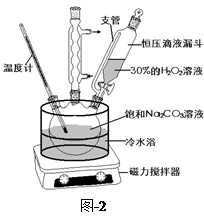
��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3��������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ��÷���ʽ��ʾ����________________________________��
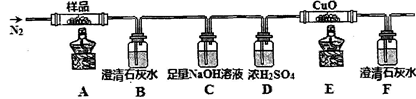
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0��1mol/LNaOH��Һ��8��0mol/LNaOH��Һ������ʯ��ˮ��0��01mol/LKMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������______________�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� | ��________________________ ��________________________ ��________________________ |
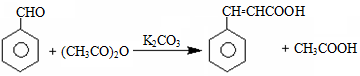
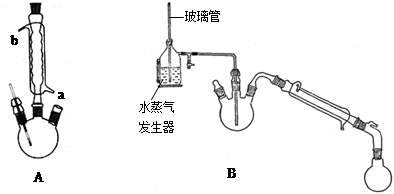
 2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��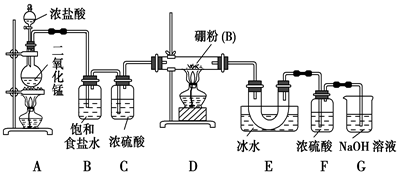
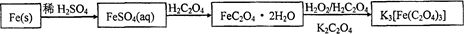
 ��Һ
��Һ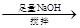
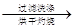 �ù���b g
�ù���b g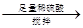
 ��������������Va mL
��������������Va mL 250 mL��Һ
250 mL��Һ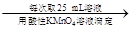 ����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��