��Ŀ����
18��Ϊ���ڡ����������ʺ���;����ij��ʦ���ö������̺�Ũ����Ϊ��Ҫԭ�ϣ����һ����ͼ��ʾ��ʵ��װ�ã�����A������ע������ͷ����Ƥ�ܣ���ͷ�Ѳ��벢������Ƥ�������н�ѧ���Իش��������⣺
��1�����е������������Ա仯�����е������Ǻ첼����ɫ����������Һ��ķ������÷�Һ©����Һ��
��2����Ƥ����ͨ��Һ©���е�����ѹǿP1����ƿ������ѹǿP2�Ĺ�ϵΪ��P1����P2������ڡ�����С�ڡ����ڡ�����������Ƥ�ܵ�Ŀ���DZ�֤��Һ©��Һ����������ѹǿ��ȣ����ڷ�Һ©����Һ��˳�����£�
��3����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ4HCl��Ũ��+Mn02$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+C12��+2H20����
���� ��1����������������Ư���Լ����������Ư���ԣ�������⻯�ط�Ӧ���ɵ⣬�����������Ȼ�̼���ֲ��Һ����÷�Һ�ķ������룻
��2��������ͷ�������ж�ѹǿ�Ĵ�С�������ѹǿ�����ڷ�Һ©���ڵ�Һ��˳�����£�
��3������������Ũ�����ڼ��������·�Ӧ�����Ȼ��̡�������ˮ��
��� �⣺��1��Cl2��ɺ첼������Ӧ�����У���û�д��������ɣ�������ɫ��������ɫ��Cl2��H2O��Ӧ���ɵ�HClO��Ư�����ã����Ա��к�ɫ������ɫ��Cl2��KI��Һ��Ӧ����I2���ʣ�CCl4��ȡ�ⵥ�ʣ���Һ�ֲ㣬���÷�Һ�ķ������룻
�ʴ�Ϊ�������Ա仯���첼����ɫ���÷�Һ©����Һ��
��2��������ͷ����Ƥ�����Ӻ�Һ©������ƿ����ѹ��ȣ�������Һ�����£�
�ʴ�Ϊ�����ڣ���֤��Һ©��Һ���ϡ�������ѹǿ��ȣ����ڷ�Һ©����Һ��˳�����£�
��3������������Ũ�����ڼ��������·�Ӧ�����Ȼ��̡�������ˮ����ѧ����ʽΪ��4HCl��Ũ��+Mn02 $\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+C12��+2H20��
�ʴ�Ϊ��4HCl��Ũ��+Mn02 $\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+C12��+2H20��
���� ���⿼������������ȡ�����ʼ��������̽����ʵ�����ƣ���ȷ�����ʹ�����������ǽ���ؼ���ע��������ʷ���ѡ���ԭ����Ŀ�ѶȲ���

 ������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺
������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺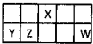
��1��д��X��ԭ�ӽṹʾ��ͼ
 ��
����2��Y��Z��W�ļ����Ӱ뾶�ɴ�С˳��ΪS2-��Cl-��Al3+�������ӷ��ű�ʾ����
��3����Y��ij���γ�������ˮ�����侻ˮԭ����Al3+3H2O?Al��OH��3�����壩+3H+�������ӷ���ʽ��ʾ����
��������ѧ��ѧԭ��������������⣺
��4����֪����C��s��+O2��g���TCO2��g������H=a kJ•mol-1��
��CO2��g��+C��s���T2CO��g������H=b kJ•mol-1��
��Si��s��+O2��g���TSiO2��s������H=c kJ•mol-1��
��ҵ�������ֹ���Ȼ�ѧ����ʽΪ2C��s��+SiO2��s��=2CO��g��+Si��s����H=��a+b-c��kJ•mol-1��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
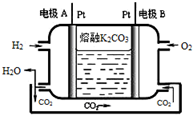
��6��������K2CO3Ϊ����ʵ�һ����������ȼ�ϵ�ع���ԭ����ͼ��ʾ��д���缫A�ĵ缫��ӦʽH2-2e-+CO32-�TCO2+H2O��
| A�� | ��ҵ�ƽ����ƣ���ⱥ��ʳ��ˮ | |
| B�� | ��ҵ���壺ijЩֲ���и�������������Ӻ���Ʒ����ȡ���ǹ�ҵ�ϻ�ȡ�����Ҫ;�� | |
| C�� | ұ���������Al2O3��ͬʱ�������ʯ��Na3AlF6����Ŀ����Ϊ�˽���Al2O3�����¶� | |
| D�� | ���Ṥҵ�����������������£��ӽӴ��ҽ����������������в����ܺ���SO2 |
��1��ѡȡ��Ҫ��ʵ��װ�ã���ȷ������˳��ΪCAB������ţ���
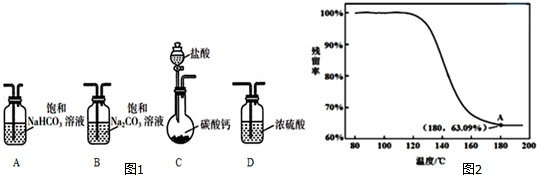
��2��Ϊȷ���ƵõĹ�����Ʒ�Ǵ�����NaHCO3��С��ͬѧ�������ʵ�鷽����
����������Ʒ��Һ�뱥�ͳ���ʯ��ˮ��Ӧ���۲�����
�ҷ���������Ʒ��Һ��BaCl2��Һ��Ӧ���۲�����
���������ⶨpH����
�����������ط�������
����������������������������
��Ϊ�ж��ҷ����Ŀ����ԣ�ijͬѧ�ô�����NaHCO3���Ƶ���Һ����BaCl2��Һ�������Ͻ���ʵ�飬������£�
NaHCO3��Һ BaCl2Ũ�� | 0.2mol•L-1 | 0.1mol•L-1 | 0.02mol•L-1 |
| 0.2mol•L-1 | ���� | ���� | �������� |
| 0.1mol•L-1 | ���� | �������� | ������ |
| 0.02mol•L-1 | �������� | ������ | ������ |
[��֪��0��l mol•L-1 NaHCO3��Һ�������c��CO32-��Ϊ0.001l mol•L-1��Ksp��BaCO3��=5.1��10-9]
��Qc=c��Ba2+����c��CO32-��=$\frac{0.2}{2}$��0.0011=1.1��10-4��5.1��10-9��
��ii���������ǣ�������������������ӷ���ʽBa2++2HCO3-=BaCO3��+CO2��+H2O��
����pH�Ʋⶨ�ı��������£�
ȡm�˵Ĺ��������ܽ���ˮ���V mL����Һ����pH�Ʋ�pH��
��Ӧ�����ʵ���ǣ���ȡ�������ķ�����NaHC03����ˮ�����V mL����Һ����pH�Ʋ�pH
�ܽ��ж�����ʵ�飬�õ�������������¶ȱ仯��������ͼ2��ʾ������A������õ��Ľ������ƵõĹ�����Ʒ�Ǵ�����NaHCO3��
��������=$\frac{ʣ����������}{ԭʼ���������}$��100%��
| A�� | 6.72L CO | B�� | 6.6g CO2 | C�� | 8 g SO2 | D�� | 9.6g H2SO4 |
| A�� | ���³�ѹ�£�0.1mol D216O�к�������������������������ΪNA | |
| B�� | �Ȼƽ��18O2����ͨ��16O2�����ֲ�ͬ�ĺ��� | |
| C�� | ��״���£�2.24LCl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA | |
| D�� | 1L2mol•L-1��Al��NO3��3��Һ�к�Al3+����Ϊ2NA |
| A�� | 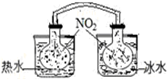 ������������������ɫ�仯���жϷ�Ӧ2NO2��g��?N2O4��g��ƽ���ƶ��ķ��� | |
| B�� | 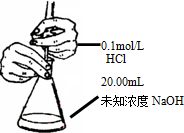 �ⶨ��ƿ�ڵ�NaOH��Ũ�� | |
| C�� | 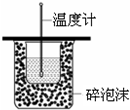 �ⶨ�к��� | |
| D�� | 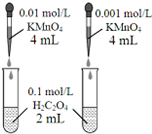 ������ɫ�����Ƚ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
| A�� | C1��C2 | B�� | C1=C2 | C�� | C1��C2 | D�� | ��ȷ�� |
 ����C��D�γɵĻ�����ĵ���ʽ
����C��D�γɵĻ�����ĵ���ʽ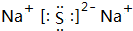 ��
��