��Ŀ����
8���л��ϳɳ��õ���-����̿��������ʹ��ʱ�������ᱻ���ʣ��������л���ȣ���Ⱦ��ʧȥ���ԣ���Ϊ�ϴ�����������������գ�һ���ɷϴ�����ȡ�Ȼ��ٵĹ���������ͼ1��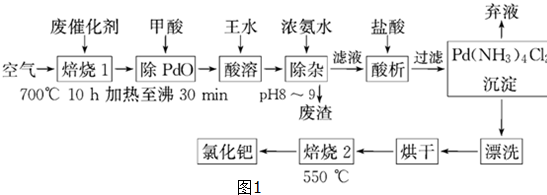
��1����������Ŀ���ǽ�PdO��ԭΪPd����Ӧ�����ɵ��������Ļ�ѧʽΪCO2��
��2��������ˮ��Ũ������Ũ���ᰴ�����1��3������õ���Һ����ת��ΪH2PdCl4�����ỹԭΪNO���÷�Ӧ�Ļ�ѧ����ʽΪ3Pd+2HNO3+12HCl�T3H2PdCl4+2NO��+4H2O��
��3���پ������ٵĻ����ʸߵ���Ҫȡ������ˮ�ܽ�IJ�����������֪��Ӧ�¶ȡ���Ӧʱ�����ˮ�������ٻ����ʵ�Ӱ����ͼ2��3��4��ʾ������ˮ�ܽ��پ�������������Ϊ80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
��4����Ũ��ˮʱ����ת��Ϊ�����Ե�[Pd��NH3��4]2+����ʱ���Ĵ�����ʽ��Fe��OH��3��д��ѧʽ����
��5��700�決��1��Ŀ���dz�ȥ����̿���л��550�決��2��Ŀ���Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��

���� ���ٴ�������ɺ�����700��ĸ����±��գ�C��Fe��Pd���л��ﱻ��������������������������м�����ᣬ�������������Ӧ�����κ�ˮ��PdO�ͼ��ᷢ��������ԭ��Ӧ����Pd�������к���Pd��SiO2���������費������ˮ����Pd��������ˮ������Һ��ͬʱ�������壬����Ũ��ˮ������ҺPH����ת��Ϊ������[Pd��NH3��4]2+��ʹ��ȫ����������Һ�м������������õ������������Ѱ���һϵ�в����õ��Ȼ��٣�
��1�����ٴ�������ɺ�����800��ĸ����±��գ�̼��������Ӧ���ɶ�����̼��Pd����������PdO��Ȼ��������м�����ᣬ���ỹԭPdO����Pd��CO2��H2O��
��2�����ݷ�Ӧ��Ͳ��������ữ�ϼ۱仯���������غ��ԭ���غ���ƽ��д��ѧ����ʽ��
��3������Pd�������뷴Ӧ�¶ȡ���Ӧʱ�䡢�پ�������ˮ�������ȵĹ�ϵȷ������������
��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+��������Һ�������ӱ�����Ϊ����������
��5�����̷�������700��ĸ����±��գ�Fe���л��ﱻ�����������������550�決��2��Ŀ�����Ѱ���
��� �⣺���ٴ�������ɺ�����700��ĸ����±��գ�Fe��Pd���л��ﱻ��������������������������м�����ᣬ�������������Ӧ�����κ�ˮ��PdO�ͼ��ᷢ��������ԭ��Ӧ����Pd�������к���Pd��SiO2���������費������ˮ����Pd��������ˮ������Һ��ͬʱ�������壬����Ũ��ˮ������ҺPH���������ӣ��õ���Һ���м������������õ�����Pd��NH3��2Cl2 ��ͨ���Ѱ���һϵ�в����õ��Ȼ��٣�
��1���ٱ�������������PdO��PdO��HCOOH����������ԭ��Ӧ����Pd��CO2��H2O����Ӧ����ʽΪPdO+HCOOH=Pd+CO2��+H2O����Ӧ�����ɵ��������Ļ�ѧʽΪCO2��
�ʴ�Ϊ��CO2��
��2���ڷ�Ӧ��PtԪ�صĻ��ϼ۱仯Ϊ0��+2��1��Pdԭ�ӵı仯��Ϊ2��NԪ�صĻ��ϼ۱仯Ϊ+5��+2��1��Nԭ�ӵı仯��Ϊ3����Ϊ��֤���ϼ���������ȣ�Ȼ�����Pdԭ�Ӹ�����ȣ���Nԭ�ӣ�Clԭ���غ㣬Ȼ���ٸ���ԭ���غ��������Ԫ�أ���3Pd+2HNO3+12HCl�T3H2PdCl4+2NO��+4H2O��
�ʴ�Ϊ��3Pd+2HNO3+12HCl�T3H2PdCl4+2NO��+4H2O��
��3������ͼ��֪���¶�Խ���ٻ�����Խ��Ӧʱ��Խ���ٻ�����Խ���پ�������ˮ��������Խ���ٻ�����Խ��90������ʱ�¶��ٸ��ٻ���������8h������ʱ����������ٻ����������پ�������ˮ��������Ϊ1��8���������پ�������ˮ�������ȣ����ٻ����ʲ�����������������80��90�棨��90�����ң�����Ӧʱ��ԼΪ8 h���پ�������ˮ��������Ϊ1��8��
�ʴ�Ϊ��80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+��������Һ�������ӱ�����Ϊ�����������ʴ�Ϊ��Fe��OH��3��
��5����700��ĸ����±��գ�C��Fe���л��ﱻ�������������������ȥ����̿���л��550�決��2��Ŀ�����Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��
�ʴ�Ϊ����ȥ����̿���л���Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��
���� ������Pd����ȡΪ���忼���˹������̣��漰��ѧʽ��ȷ����������ԭ��Ӧ��ͼ�������֪ʶ�㣬��ȷ���ʵ������ǽⱾ��ؼ���ע��������Ϸ������֪��ÿһ���漰�ķ�Ӧ�����ʷ���ķ�����֪ʶ�㣬���Ԫ���غ���������Ŀ�Ѷ��еȣ�

| A�� | 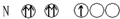 | B�� | 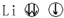 | C�� | 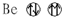 | D�� | 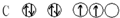 |
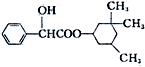 �������������ɳ�Ѫ��ƽ����������Ѫ�ܵĹ��ܣ���ṹ��ʽ��ͼ�����жԸ����ʵ������У���ȷ���ǣ�������
�������������ɳ�Ѫ��ƽ����������Ѫ�ܵĹ��ܣ���ṹ��ʽ��ͼ�����жԸ����ʵ������У���ȷ���ǣ�������| A�� | ���������ڷ����� | |
| B�� | ���л��ﲻ�ܱ�������ȩ | |
| C�� | ����ʽΪC17H23O3 | |
| D�� | 1mol������������2 mol NaOH������Ӧ |
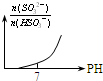
| A�� | ֻ�дﵽƽ��ʱ������O2������������NO������֮�Ȳ�Ϊ5��4 | |
| B�� | ����λʱ������xmolNO��ͬʱ������xmolNH3����Ӧ�ﵽƽ��״̬ | |
| C�� | �ﵽƽ��״̬��NH3��O2��NO��H2O��g�������ʵ������ֲ��� | |
| D�� | �ﵽƽ��״̬ʱ�������������������Ӧ�������� |
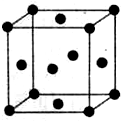 ��1��NF3������Nԭ�ӵ��ӻ��������Ϊsp3���÷��ӵĿռ乹��Ϊ������
��1��NF3������Nԭ�ӵ��ӻ��������Ϊsp3���÷��ӵĿռ乹��Ϊ������
 ��
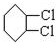
��

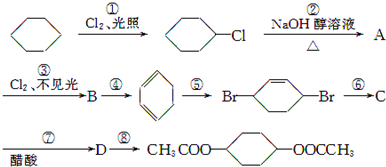
 ��C��
��C�� ��
��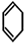 +2NaCl+2H2O
+2NaCl+2H2O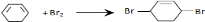
 ��
��