��Ŀ����
1����ⷨ�ٽ����ʯ����Ҫ�ɷ���Mg2SiO4���̶�CO2�IJ��ֹ����������£���֪��Mg2SiO4��s��+4HCl��aq��?2MgCl2��aq��+SiO2 ��s��+2H2O��l����H=-49.04kJ•mol-1
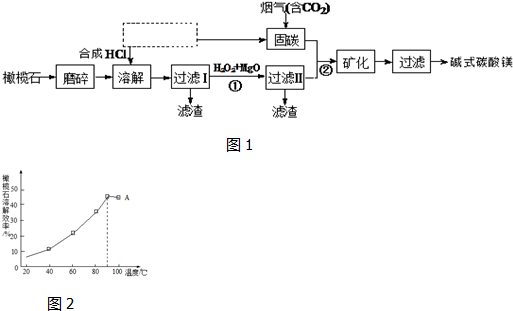
��1�����ʯ�������Mg9FeSi5O20�������������ʽ�ɱ�ʾΪ9MgO•FeO•5SiO2��
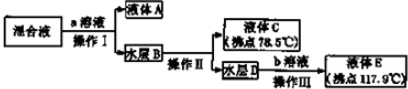
��2��ͼ1�������Ҫ����һ����ҵ����������Ϊ�ȼҵ��
��3������������Ҳ����������̼������bc��������ĸ��
a��CaCl2 b��H2NCH2COONa c����NH4��2CO3
��4����ͼ2��֪��90�������A�ܽ�Ч���½���������ԭ��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��ͣ�
��5�����ˢ�������Һ�к���Fe2+����������ӷ���Ϊȡ������Һ������KSCN��Һ����������ˮ��Һ���ɫ֤�����������ӻ�ȡ������Һ���Թ��У��μӼ���K3[Fe��CN��6]��Һ����������ɫ�������ɣ���֤����Һ����Fe2+��
��6�����̢�Ϊ��ȥ��Һ�е����ʣ�д���ó��ӹ������漰��Ӧ�����ӷ���ʽ2Fe2++H2O2+2H+=2Fe3++2H2O��2Fe3++3MgO+3H2O=2Fe��OH��3+3Mg2+��
���� ��1���������ǹ��ɵؿ���ʯ����Ҫ�ɷ֣���ѧ�ϳ��ö�����������������ʽ��ʾ����ɣ����磺þ���ʯ��Mg2SiO4������2MgO•SiO2��ʾ���ɿ�����д��Ҫ��֤ԭ�����������ϼ۲��䣬�����ϼ۷ֱ�д��������Ļ�ѧʽ�����ж��ԭ�ӣ���ǰ�����ϵ����ʹ֮��Ϊ�������ݴ˽��з������
��2����ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռ
��3�������ܺͶ�����̼֮�䷴Ӧ�����������̶�������̼���ش�
��4���¶ȶԻ�ѧ��Ӧƽ���ƶ���Ӱ��֪ʶ���ش�
��5���������ӵļ�����KSCN����ˮ��
��6�����̢�Ϊ��ȥ��Һ�е����ʣ���ӦΪ�������������������ӣ�����þ�������ӷ�Ӧ��������������þ���ӣ�
��� �⣺��1�����ݹ�����д��������Ĺ��ɣ�Mg9FeSi5O20�����������ʽ�ɱ�ʾΪ9MgO•FeO•5SiO2���ʴ�Ϊ��9MgO•FeO•5SiO2��
��2����������ͼ����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH��aq��+CO2 ��g��=NaHCO3 ��aq������ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռȱ���ռ����ȡ���̣����ɵ��Ȼ����ǵ���Ȼ�����Һ���ɵ�������������Ӧ�õ���
�ʴ�Ϊ���ȼҵ��
��3�������������У�NH3•H2O��Na2CO3 ���ԺͶ�����̼��������̼����李�̼�����ƣ�����������̼�����Լ���
�ʴ�Ϊ��bc��
��4��ͼ����ʾ�����Լ����߱仯֪����20min���ܽ�ﵽƽ�⣬���÷�Ӧ�Ƿ��ȣ����£�ƽ�������ƶ������ܽ�Ч�ʽ��ͣ�
�ʴ�Ϊ��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��ͣ�
��5�����ˢ�������Һ�к���Fe2+����������ӷ���Ϊȡ������Һ������KSCN��Һ����������ˮ��Һ���ɫ֤�����������ӣ���ȡ������Һ���Թ��У��μӼ���K3[Fe��CN��6]��Һ����������ɫ�������ɣ���֤����Һ����Fe2+��
�ʴ�Ϊ��ȡ������Һ������KSCN��Һ����������ˮ��Һ���ɫ֤�����������ӻ�ȡ������Һ���Թ��У��μӼ���K3[Fe��CN��6]��Һ����������ɫ�������ɣ���֤����Һ����Fe2+��
��6�����̢�Ϊ��ȥ��Һ�е����ʣ���ӦΪ�������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O������þ�������ӷ�Ӧ��������������þ���ӣ���Ӧ�����ӷ���ʽΪ 2Fe3++3MgO+3H2O=2Fe��OH��3+3Mg2+��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��2Fe3++3MgO+3H2O=2Fe��OH��3+3Mg2+��
���� ������һ����ѧ��������ϵĹ��������⣬�����ڿ��Ե��ȵ㣬ע��֪ʶ��Ǩ�ƺ����Ӧ���ǽ���Ĺؼ����Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ϩ��ʵ��ʽC2H4 | B�� | �Ҵ��Ľṹ��ʽC2H6O | ||
| C�� | ���Ȼ�̼�ĵ���ʽ��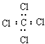 | D�� | 3��3��4-��������ķ���ʽC9H20 |
| A�� | ����ʹ���Ը��������Һ��ɫ | |
| B�� | �ҿ�����ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ | |
| C�� | ������ˮ���Է���ȡ����Ӧ | |
| D�� | ����ϡ���������¿������ᷢ��ȡ����Ӧ |
| A�� | ԭ��������a��b��c | B�� | ���Ӱ뾶��Yn-��Zm- | ||
| C�� | a-b=m-n | D�� | ԭ�Ӻ���������������X��Y��Z |
| A�� | �������ʵ�����KHC2O4��H2C2O4����ˮ�����Һ��2c��K+��=c��HC2O4-��+c��H2C2O4�� | |
| B�� | pH��ȵĢ�NH4Cl���ڣ�NH4��2SO4����NH4HSO4������Һ�У�c��NH4+����С����=�ڣ��� | |
| C�� | 0.1mol/LCH3COONa��Һ��0.15 mol•L-1HCl�������ϣ�c��Cl-����c��H+����c��Na+����c��CH3COO-����c��OH-�� | |
| D�� | 0.1 mol•L-1��KHA��Һ����pH=10��c��K+����c��A2-����c��HA-����c��OH-�� |
| A�� | Һ������������� | |
| B�� | �����������Ʊ��轺��ľ�ķ������ԭ�� | |
| C�� | ���ȵĴ�����Һϴ������ | |
| D�� | ��������������ˮ��ɱ������ |
| A�� | ��ϩ�뻷���飨C3H6����Ϊͬϵ�� | |
| B�� | ��ϩ�;���ϩ���ܺ���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ | |
| C�� | Ƥ��մ��ŨHNO3��ɻ�ɫ�������ñ���NaOH��Һ��ϴ | |
| D�� | ���ᡢ���������2-�ǻ���ȩ��CH3CHOHCHO����Ϊͬ���칹�� |
 �����ǰ״��е���Ҫ�ɷ֣�
�����ǰ״��е���Ҫ�ɷ֣�