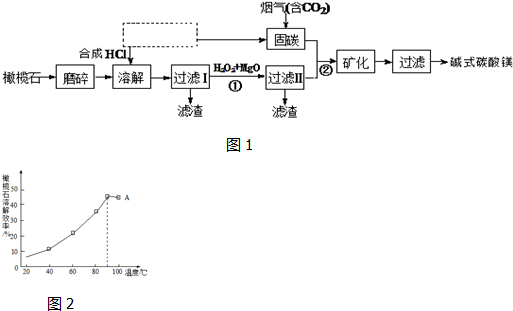
��Ŀ����
10��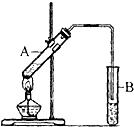 �����ǰ״��е���Ҫ�ɷ֣�
�����ǰ״��е���Ҫ�ɷ֣���1���״�ʹ��ɫʯ����Һ��ɺ�ɫ��˵������������ԣ�
��2��ʵ��������ͼ��ʾװ����ȡ�������������Թ�A�м�����Լ����Ҵ���Ũ����ͱ����ᣮ���Թ�B�м�����Լ��DZ���̼������Һ�����ܲ�������Һ�е�ԭ���Ƿ�ֹ������
��3��A�з�Ӧ�Ļ�ѧ����ʽ��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
��4�����Թ�B�з��������������ʵ�鷽���Ƿ�Һ��
���� ��1����������Һ�е���������ӣ���Һ�����ԣ��ܹ�ʹ��ɫʯ����Һ��죻
��2����ȡ����������Ҫ�õ��Ҵ��������Ũ����ñ���̼������Һ�����������������ܲ�����Һ����������������
��3��A���Ҵ���������Ũ���������·���������Ӧ��������������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��4���������������ڱ���̼������Һ�����Һ�ֲ㣬��ͨ����Һ�������������������
��� �⣺��1���״���Ҫ�ɷ�Ϊ���ᣬ�����ܹ�����������ӣ���ҺΪ���ԣ��ܹ�ʹ��ɫʯ����Һ��ɺ�ɫ��
�ʴ�Ϊ����ɫ��
��2����ȡ����������ԭ��Ϊ�Ҵ����������̼������Һ�ܹ������Ҵ����к����ᣬ�����������ڱ���̼������Һ�е��ܽ�Ƚ�С��ͨ���ñ���̼������Һ��������������Ϊ�˱��ⷢ�����������������������ĵ��ܲ�������Һ���У�
�ʴ�Ϊ���Ҵ�������̼������Һ����ֹ������
��3��A���Ҵ������ᷢ��������Ӧ�ķ���ʽΪ��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
��4���������������ڱ���̼������Һ������Һ��ֲ㣬��ͨ����Һ�������������������
�ʴ�Ϊ����Һ��
���� ���⿼�������������Ʊ���������Ŀ�ѶȲ���ע���������������ķ�Ӧԭ������ȷŨ���ᡢ����̼������Һ��ʵ���е����ã�Ϊ��Ƶ���㣬����������ѧ���ķ�����������ѧʵ��������

| A�� | Y��X��Z��W�ֱ����γ����ֻ����� | |
| B�� | Z��W�γɵ�Z2W�ͻ�������ˮ��Һ��Z+������W2-���Ӹ�����Ϊ2��1 | |
| C�� | Y��Z��W�������γ�ˮ��Һ�ʼ��ԵĻ����� | |
| D�� | ������X Y2��X YW��X W2���Ź��ۼ��ļ����������۷е����� |
| A�� | ��ѧ��Ӧ�ﵽ��ѧƽ��״̬�����淴Ӧ������ȣ���ָͬһ���ʵ��������ʺ�����������ȣ����ò�ͬ���ʱ�ʾʱ����Ӧ���ʲ�һ����� | |
| B�� | ��״���£�1L������ȫȼ������CO28L | |
| C�� | 2.4gMg������O2������N2��ȫ��Ӧ��ת�Ƶ���������0.2NA | |
| D�� | 1L 1mol•L-1 CH3COOH��Һ�У�����CH3COO-��CH3COOH������ΪNA |
| A�� | ��ϩ���廯�����������뱽���巴Ӧ���屽 | |
| B�� | ������ˮ�����ɰ���������۷������ɾƾ� | |
| C�� | �Ҵ���ˮ����ϩ���Ҵ���������ˮ���������� | |
| D�� | �����������һ�ȼ����뱽�����ᷴӦ�������� |
��1��������ijͬѧ���й����ʽ��з�����б���
| �� | �� | �� | ���������� | ���������� | |
| ��һ�� | Na2CO3 | H2SO4 | NaHCO3 | CaO | CO2 |
| �ڶ��� | NaOH | HCl | NaCl | Na2O | CO |
| ������ | NaOH | CH3COOH | CaF2 | Al2O3 | SO2 |
��2���������Һ�ı��������Ƿ�ɢ����ֱ����С�����������Һ�����õķ����ǹ۲��Ƿ��ܷ������������
��3������3����Ӧ����Ҫ����д�������
��2Na2O2+2H2O�T4NaOH+O2����Ӧ�У�ÿ����1mol Na2O2����16g O2��
��2NaHCO3�TNa2CO3+H2O+CO2����Ӧ�У�ÿ����168g NaHCO3�����������22.4L CO2��
��Cl2+H2O�THCl+HClO��Ӧ�У������ÿ����22.4L Cl2��ת��1mol���ӣ�
��4����һ���ܱ������з���M��N��Q��P�������ʣ���һ�������·�����ѧ��Ӧ��һ��ʱ�����й��������±�����Ҫ��ش��������⣺
| ���� | M | N | Q | P |
| ��Ӧǰ������g�� | 50 | 1 | 3 | 12 |
| ��Ӧ��������g�� | X | 26 | 3 | 30 |
| A�� | 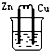 | B�� | 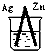 | C�� | 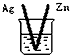 | D�� | 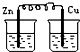 |