��Ŀ����
2���������Ƿ��Ϳɵ����ᣬΪ�о�����ķ�����ɺͻ�������������ʵ�飺��1����ȡ����0.90g����ij״����ʹ����ȫ��������֪��ͬ״����ͬ�������Ϊ0.02g�����������Է�������Ϊ90��
��2��������������������������ȼ��ֻ����CO2��H2O��g������ȫ������ʯ������ʱ����ʯ������1.86g�������˲���ͨ�����ʯ��ˮ�У�������3.00g��ɫ������������ķ���ʽΪC3H6O3��
��3����ȡ0.90g���ᣬ�������������Ʒ�Ӧ������H2 224mL������£�����������Na2CO3��Ӧ����112mL CO2������£���������Ľṹ��ʽ������CH2��OH��CH2COOH��
 ��
����4����������������Ũ������ȵ������·�Ӧ����һ��������Ԫ���Ļ�����������Ľṹ��ʽ
 ������Ԫ�����Ľṹ��ʽΪ
������Ԫ�����Ľṹ��ʽΪ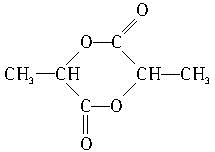 ��
��
���� ��1����Է�������֮�ȵ����ܶ�֮�ȣ�
��2����ʯ�����ص�����Ϊ������̼��ˮ��������3.00g��ɫ����Ϊ̼��ƣ�
��3���ǻ����Ȼ������Ʒ�Ӧ���Ȼ�����̼���Ʒ�Ӧ��
��4��Ũ������������������ӵ�CH2��OH��CH2COOH�ܷ�Ӧ���ɰ�Ԫ��״�����
��� �⣺��1����Է�������֮�ȵ����ܶ�֮��=$\frac{0.90g}{0.02g}$=45���������Է�������Ϊ90���ʴ�Ϊ��90��
��2��3.00g��ɫ����Ϊ̼��ƣ����ʵ���Ϊ0.03mol����������ʵ���Ϊ0.01mol��0.01mol������ȫȼ������CO2 0.03 mol������H2O��
$\frac{1.86g-0.03mol��44g/mol}{18g/mol}$=0.03 mol������һ�������������3��̼ԭ�Ӻ�6��Hԭ�ӣ���������Է�������Ϊ90���ʺ���ԭ����Ϊ$\frac{90-3��12-6}{16}$=3����������ķ���ʽΪC3H6O3���ʴ�Ϊ��C3H6O3��
��3���ǻ����Ȼ������Ʒ�Ӧ��H2 224mL�����ʵ���Ϊ0.01mol���Ȼ�����̼���Ʒ�Ӧ��112mL CO2�����ʵ���Ϊ0.005mol��˵���ǻ����Ȼ��ĸ�����ȣ������Է�������Ϊ90����֪����Ľṹ��ʽ������CH2��OH��CH2COOH�� ���ʴ�Ϊ��CH2��OH��CH2COOH��
���ʴ�Ϊ��CH2��OH��CH2COOH�� ��
��
��4��Ũ������������������ӵ�CH2��OH��CH2COOH�ܷ�Ӧ���ɰ�Ԫ��״�����Ũ������������������ӵ� �ܷ�Ӧ������Ԫ��״�������û�����Ľṹ��ʽΪ
�ܷ�Ӧ������Ԫ��״�������û�����Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
���� ���⿼�����л���ṹ�����ʡ��л���Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ��ܹ�������д������Ӧ�Ļ�ѧ����ʽ��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д���1��K+��Fe2+��SO42-��ClO- ��2��K+��Al3+��Cl-��HCO3-
��3��ClO-��Cl-��K+��OH- ��4��Fe3+��Cu2+��SO42-��Cl-
��5��Na+��K+��AlO2-��HCO3- ��6��Ca2+��Na+��SO42-��CO32-
��ˮ��Һ���ܴ���������ǣ�������
| A�� | ��1���ͣ�6�� | B�� | ��3���ͣ�4�� | C�� | ��2���ͣ�5�� | D�� | ��1���ͣ�4�� |
| A�� | �۵㣺CO2��H2O��SiO2��KCl | B�� | ��ԭ�ԣ�S2-��I-��Br-��Cl- | ||
| C�� | ���ԣ�H3PO4��H2SO4��HClO4��H2SiO3 | D�� | �ȶ��ԣ�H2O��NH3��PH3��SiH4 |
| A�� | NaCl | B�� | C | C�� | Fe | D�� | Ar |
| A�� | ԭ�����Һ��c��Na+ ��=6mol/L | B�� | ������Һ��c��H+��=4mol/L | ||
| C�� | �����������й�ת��8mol���� | D�� | ����õ���Cu�����ʵ���Ϊ2mol |
| A�� | ��ɡ����ꡱ��������� | B�� | ˮ��Һ�ʼ��� | ||
| C�� | ������Ʒ����Һ��ɫ | D�� | ����ʹ�����ʯ��ˮ����� |
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
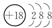 ��
����2���������γ��������������Ԫ����������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������Ϊ���û�ѧʽ��ʾ��KOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
��4����Ԫ�����Ԫ�����ߺ˵����֮����26��
��5����д���ڵ��⻯����������Ļ�ѧ����ʽ4NH3+5O2
 4NO+6H2O��
4NO+6H2O�� 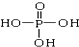 ��
�� ���������Һ��0.1mol•L-1��NaOH��Һ�ζ���������NaOH��Һ 42.00mL��50.00mLʱ����һ���ζ��յ㣮��ش�
���������Һ��0.1mol•L-1��NaOH��Һ�ζ���������NaOH��Һ 42.00mL��50.00mLʱ����һ���ζ��յ㣮��ش�