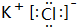��Ŀ����
12��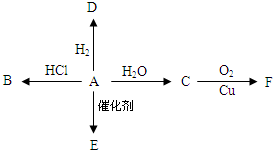 ��ͼ��ʾ����֪�л���A�IJ������Ժ���һ�����ҵ�ʯ�ͻ�����չˮƽ��A���Է�����ͼһϵ�еķ�Ӧ����Ҫ��ش���������
��ͼ��ʾ����֪�л���A�IJ������Ժ���һ�����ҵ�ʯ�ͻ�����չˮƽ��A���Է�����ͼһϵ�еķ�Ӧ����Ҫ��ش�����������1��д�����ʵĽṹ��ʽA��CH2=CH2��B��CH3CH3��D��CH3CH2OH��
��2��д�����ʵ�����C���Ҵ��� E��A�����ӳɾۺ��Ժ�IJ����E�������Ǿ���ϩ��
��3��д����ѧ��Ӧ����A��D�ӳɷ�Ӧ��
��4��C�й����ŵ��������ǻ���
��5��д����ѧ��Ӧ����ʽ��A��D��CH2=CH2+H2$\stackrel{����}{��}$CH3CH3��C��F��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ��A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2����ϩ�����������ӳɷ�Ӧ����DΪCH3CH3����ϩ��HCl�����ӳɷ�Ӧ����BΪCH3CH2Cl����ϩ��ˮ�����ӳɷ�Ӧ����CΪCH3CH2OH����ϩ�����Ӿ۷�Ӧ���ɸ߷��ӻ�����EΪ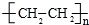 ���Ҵ�������FΪCH3CHO���ݴ˽��
���Ҵ�������FΪCH3CHO���ݴ˽��
��� �⣺��A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2����ϩ�����������ӳɷ�Ӧ����DΪCH3CH3����ϩ��HCl�����ӳɷ�Ӧ����BΪCH3CH2Cl����ϩ��ˮ�����ӳɷ�Ӧ����CΪCH3CH2OH����ϩ�����Ӿ۷�Ӧ���ɸ߷��ӻ�����EΪ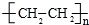 ���Ҵ�������FΪCH3CHO��
���Ҵ�������FΪCH3CHO��
��1����������ķ�����֪��A�ĽṹʽΪCH2=CH2��BΪCH3CH3��DΪCH3CH2OH��
�ʴ�Ϊ��CH2=CH2��CH3CH3��CH3CH2OH��
��2��CΪCH3CH2OH�����������Ҵ���EΪ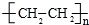 ���������Ǿ���ϩ��
���������Ǿ���ϩ��
�ʴ�Ϊ���Ҵ�������ϩ��
��3����������ķ�����֪��A��DΪ�ӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
��4��CΪCH3CH2OH��C�й����ŵ��������ǻ����ʴ�Ϊ���ǻ���
��5��A��D�Ļ�ѧ��Ӧ����ʽΪCH2=CH2+H2$\stackrel{����}{��}$CH3CH3��C��F�Ļ�ѧ��Ӧ����ʽΪ2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��CH2=CH2+H2$\stackrel{����}{��}$CH3CH3��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
���� ���⿼���л�����ƶϣ��漰ϩ��������������ȩ����֮���ת�����Ƚϻ�����ּ�ڿ���ѧ���Ի���֪ʶ�����գ�ע�����֪ʶ��ȫ�����գ�

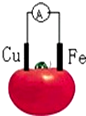
| A�� | ͭƬΪ���� | B�� | ��Ƭ�Ϸ�����ԭ��Ӧ | ||
| C�� | ��������Ƭ�ص�������ͭ�� | D�� | ͭƬ�ϵĵ缫����ʽ��2H++2e-=H2�� |
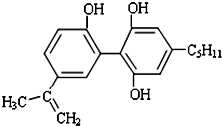 Ϊ��֤�����ɼ��Ĺ�������ƽ�����˷ܼ���һ����Ҫ����Ĺ�����ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵��������ǣ�������
Ϊ��֤�����ɼ��Ĺ�������ƽ�����˷ܼ���һ����Ҫ����Ĺ�����ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵��������ǣ�������| A�� | ���л�������FeCl3��Һ������ɫ��Ӧ | |
| B�� | һ��������1mol����������7molH2�����ӳɷ�Ӧ | |
| C�� | ���л�����������е�ԭ�Ӳ�������ͬһƽ�� | |
| D�� | ���л����DZ��ӵ�ͬϵ�� |
| A�� | ������ܽ� | B�� | ����ʹʯ����Һ��� | ||
| C�� | �����ж������Ȼ� | D�� | ��һ���������ܷ���������Ӧ |
��ȡ����Ӧ���ڼӳɷ�Ӧ����������Ӧ����������Ӧ����ˮ�ⷴӦ��
| A�� | �١��ڡ��ۡ��� | B�� | �ڡ��ۡ��ܡ��� | C�� | �١��ڡ��ܡ��� | D�� | ȫ�� |
| A�� | v��A��=4 mol•L-1•min-1 | B�� | v��B��=5 mol•L-1•min-1 | ||
| C�� | v��C��=5 mol•L-1•min-1 | D�� | v��D��=6 mol•L-1•min-1 |
| A�� | H2S | B�� | NaOH | C�� | Na2CO3 | D�� | KSCN |