��Ŀ����
����Ŀ�������£���0.100mol��L1��NaOH��Һ�ֱ�ζ���Ϊ20.00mL0.100mol��L1��HCl��Һ�ʹ�����Һ���ζ�������ͼ��ʾ������˵����ȷ����

A.���ʾ���ǵζ����������
B.pH=7ʱ���ζ��������ĵ�V��NaOH����20.00mL
C.V��NaOH����20.00mLʱ��������Һ��c��Cl������c��CH3COO����
D.V��NaOH����10.00mLʱ��������c��Na+����c��CH3COO������c��H+����c��OH����
���𰸡�C
��������
A. 0.100mol��L-1��HCl��Һ�ʹ�����Һ�������������ᣬ���ڵ���ƽ�⣬�������pH��С�Ģ��ʾ���ǵζ���������ߣ�A�����
B. ����������������ǡ����ȫ��Ӧʱ���γɴ�������Һ��������ˮ��ʹ��ҺpH>7������pH =7ʱ���ζ��������ĵ�V(NaOH)<20.00mL��B�����
C. V(NaOH)= 20.00mLʱ�����ǡ����ȫ��Ӧ����ΪCH3COO-ˮ������ģ�����������Һ��c(Cl-����c(CH3COO-����C����ȷ��
D. V(NaOH)=10.00mLʱ�����ɵĴ�������ʣ�����Ũ����ȣ����ڴ���ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ�������Һ��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)��D�����
��ѡC��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�����Ŀ����֪��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H��Q����ƽ�ⳣ�����¶ȱ仯���±���ʾ��
CO2(g)��H2(g) ��H��Q����ƽ�ⳣ�����¶ȱ仯���±���ʾ��
�¶�/�� | 400 | 500 | 850 |
ƽ�ⳣ�� | 9.94 | 9 | 1 |
��ش��������⣺
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___���÷�Ӧ��Q__0(����������������)��
��2��850��ʱ�����Ϊ10L��Ӧ���У�ͨ��һ������CO��H2O(g)������������Ӧ��CO��H2O(g)Ũ�ȱ仯��ͼ��ʾ����0��4 minʱƽ����Ӧ����v(CO)��__��
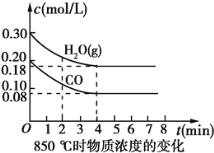
��3��400��ʱ��ѹǿ�㶨���ܱ������н���������Ӧ���ÿ��淴Ӧ�ﵽƽ��ı�־��__(����ĸ)��
A��v��(H2)��v��(CO)
B�������������������ʱ����仯
C�����������ܶȲ�����ʱ��仯
D��CO��H2O��CO2��H2�ķ�����֮��Ϊ1��1��1��1
��4������500��ʱ���У���CO��H2O(g)����ʼŨ�Ⱦ�Ϊ0.020molL-1���������£�CO�����ת����Ϊ__��
��5������850��ʱ���У�ijʱ��ʱ���CO(g)��H2O(g)��CO2(g)��H2(g)�����ʵ����ֱ�Ϊ1mol��0.5mol��0.6mol��0.6mol����ʱV��__V����(��������������������=��)
��6������850��ʱ���У�����ʼʱCO��H2O(g)��Ϊ1mol������ˮ�������������Ϊx��ƽ��ʱCO��ת����Ϊy�����Ƶ�y��x�仯�ĺ�����ϵʽΪ__��
����Ŀ��úȼ���ŷŵ���������SO2��NO���γ����ꡢ��Ⱦ����������NaClO2��Һ��Ϊ���ռ���ͬʱ���������������������ش��������⣺
��1��NaClO2��ClԪ�صļ�̬Ϊ_______��
��2���ڹ��ݷ�Ӧ����ͨ�뺬�к���SO2��NO����������Ӧ�¶�Ϊ323 K��NaClO2��ҺŨ��Ϊ5��103mol��L1 ����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±�:
���� | SO42 | SO32 | NO3 | NO2 | Cl |
c/��mol��L1�� | 8.35��104 | 6.87��106 | 1.5��104 | 1.2��105 | 3.4��103 |
����NaClO2��Һ��������Ҫ��Ӧ�У��μӷ�Ӧ��n��ClO2-����n��NO��=_________������ѹǿ��NO��ת����______���������������������������������
���������շ�Ӧ�Ľ��У����ռ���Һ��pH��_______����������������������������������
����ʵ������֪������Ӧ����______������Ӧ���ʣ���������������С��������ԭ���dz���SO2��NO�������еij�ʼŨ�Ȳ�ͬ����������___________��
��3���ڲ�ͬ�¶��£�NaClO2��Һ���������ķ�Ӧ�У�SO2��NO��ƽ���ѹpc����ͼ��ʾ��
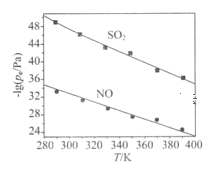 =
=
����ͼ������֪����Ӧ�¶����ߣ�����������Ӧ��ƽ�ⳣ����____��������������������������С������
�ڷ�ӦClO2+2SO32===2SO42+Cl��ƽ�ⳣ��K����ʽΪ___________��



