��Ŀ����
��18�֣��������ʽṹ��֪ʶ����������⡣
��1����һ�����ܽ���B��N֮��ĵڶ�����Ԫ���� (��Ԫ�ط���)��
��2��������[TiCl(H2O)5]2�����������ӻ��ϼ�Ϊ���� ��������Ļ�ѧʽΪ ��
��3��BF3��һ����ˮ�γ�(H2O)2��BF3����Q��Q��һ�������¿�ת��ΪR��
Q R
�پ���Q�в����ڵ�������Ϊ ������ĸ����
A�����ۼ� B�����Ӽ� C����λ�� D�����»��� E�����
��R�������ӵĿռ乹��Ϊ ��
��4����ɰ�Ǻ��ᾧˮ���������ƣ���������Xm������B��O��H����Ԫ�أ������ģ������ͼ3��ʾ����m�� �������֣���
��5�������Ȼ��ѡ�̼���ơ��������Σ���NaN3����ԭ�ϣ���������̼�����ѻ������ṹ����̼ԭ��ȡ�������Ѿ������ṹ����ͼ4�����ж���ĵ�ԭ�ӣ�����̼�����ѻ�����Ļ�ѧʽΪ ��
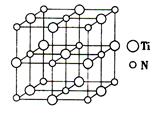
ͼ3 ͼ4
��6�����ֻ�ѧ���ļ��ܼ��±���
| ��ѧ�� | Si��O | Si��Cl | H��H | H��Cl | Si��Si | Si��C |
| ����/ kJ��mol��1 | 460 | 360 | 436 | 431 | 176 | 347 |
�ڹ�ҵ�ϸߴ����ͨ�����з�Ӧ��ȡ��SiCl4 (g) + 2H2(g) ����Si(s) + 4HCl(g)
����÷�Ӧ�ķ�Ӧ�ȡ�H ��___________ kJ/mol��
��1��Be��C��O ��2�� +3 ��Cl����H2O ��3��B�������� ��4��2 ��5�� Ti4CN3
��6���������� 360��4+436��2��(176��2+431��4)��+236
���������������1��ͬ�����������ҵ���������������BeԪ�ص�2s������Ӵ���ȫ����״̬���ȶ���ǿ�����BeԪ�صĵ�һ�����ܴ���BԪ�صĵ�һ�����ܡ���Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ�������Ԫ�ص�һ������С�ڵ�Ԫ�صĵ�һ�����ܣ���˵�һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O��
��2��ˮ����������0�۵ģ���Ԫ���ǣ�1�ۣ���������[TiCl(H2O)5]2�����������ӻ��ϼ�Ϊ��3�ۣ��ܹ��ṩ�¶Ե��ӵ������壬��˸�������������Ļ�ѧʽΪCl����H2O��
��3���ٸ��ݾ���Q�Ľṹ��֪�������д�����������ۼ������»���������B��O֮�仹����λ�����������������ӣ����û�����Ӽ�����ѡB��
��R����������ˮ�������ӣ�������ԭ�ӵļ۲���Ӷ�����4����������1���¶Ե��ӣ������ռ乹��Ϊ�����Ρ�
��4���������ģ�� ��֪1��3��5��6������ԭ�ӣ�2��4����Bԭ�ӣ��۲�ģ�ͣ���֪Xm-�ǣ�H4B4O9��m-�����ݻ��ϼ�HΪ+1��BΪ+3��OΪ-2���ɵ�m��2��
��֪1��3��5��6������ԭ�ӣ�2��4����Bԭ�ӣ��۲�ģ�ͣ���֪Xm-�ǣ�H4B4O9��m-�����ݻ��ϼ�HΪ+1��BΪ+3��OΪ-2���ɵ�m��2��
��5�����ݾ����ṹ��֪������̼ԭ�ӵĸ�����8�� ��1����ԭ�Ӹ�����6��
��1����ԭ�Ӹ�����6��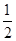 ��3��Tiԭ�Ӹ�����12��
��3��Tiԭ�Ӹ�����12��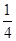 +1��4�����Ծ����Ļ�ѧʽΪTi4CN3��
+1��4�����Ծ����Ļ�ѧʽΪTi4CN3��
��6����̼����赥���γɵľ���������ԭ�Ӿ��壬����̼ԭ�Ӱ뾶С�ڹ�ԭ�ӣ����̼����ķе���ڵ��ʹ�ķе㡣���Ȼ�̼�γɵľ����Ƿ��Ӿ��壬���������γɵľ�����ԭ�Ӿ��壬������Ȼ���ķе���ڶ�������ķе㡣
�ڻ�ѧ��Ӧ����ʽ�еķ�Ӧ��=��Ӧ��ļ���֮�ͣ�������ļ���֮�ͣ����������1����ԭ���γ�2�����ۼ������Ը��ݷ���ʽ��֪��H��360kJ/mol��4��436kJ/mol��2��176kJ/mol��2��431kJ/mol��4����236 kJ/mol��
���㣺��������ܡ���λ������ѧ�����������㡢���������Լ���Ӧ�ȼ���

��4�֣��±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
| | | | |||||
| | | | a | | | | |
| | b | | c | | | d | |
��1��Ԫ��b�Ļ�̬ԭ�ӵ����Ų�ʽΪ��������
��2����������8��Ԫ�ذ������۵�ߵ͵�����˳������ͼ��������š�8������������Ԫ�ط��ţ������е縺������������������ͼ�е���ţ���
��3��Ԫ��a��c�ֱ���Ԫ��d�γɵĻ������У��۵�ϸߵ��ǣ��ѧʽ����
�±���Ԫ�����ڱ���һ���֣����������Ԫ�أ�������и�С�⡣
 | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 2 | | | | C | N | O | |
| 3 | Na | | Al | Si | | S | Cl |
21. ��3�����н�������ǿ��Ԫ���� ����Ԫ�����ƣ���
22. C��N��Oԭ�Ӱ뾶��С�����˳����� ��
23. ��3��������̬�⻯�����ȶ����� ���ѧʽ����
24. Si�Ǵ���������������ҪԪ��֮һ���������ﻯѧʽ�� ��
25. ���Ǵ���Ȼ��������ı������һ����;�Ƿ������ȷ�Ӧ��ұ��ijЩ���۽�����д��
�÷�Ӧ��һ����ѧ����ʽ ��
26. SԪ���γɵ��⻯������������SO2������������� ��
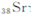 )Ԫ�ع㷺�����ڿ�Ȫˮ��,��һ������������Ԫ��,��Ԫ�����ڱ�����
)Ԫ�ع㷺�����ڿ�Ȫˮ��,��һ������������Ԫ��,��Ԫ�����ڱ�����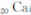
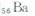 ͬ���ڵڢ�A��?
ͬ���ڵڢ�A��?
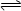 C2D4��g���ﵽƽ��״̬�ı�־�� ��
C2D4��g���ﵽƽ��״̬�ı�־�� �� 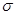 ����
����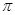 ����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)��
����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)��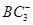 ������Bԭ�ӹ�����ӻ�����Ϊ__________��
������Bԭ�ӹ�����ӻ�����Ϊ__________��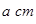 ���þ�����ܶ�Ϊ________
���þ�����ܶ�Ϊ________ g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��
g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��