��Ŀ����
����Ŀ����Զ��ijУ�Ļ�ѧ��ȤС�龭����̽��ʵ�飺
��һ��Ϊ��̽��һ�������ܷ�Na2O2��ȫ���գ����������ʵ�顣װ�����£�����װ��ʡ������

����������֪����2NO+Na2O22NaNO2
�����������£�NO��NO2������KMnO4��Һ��Ӧ����NO3������
�ش��������⣺
��1������a���ƣ�________����
��2��Bƿ��װ�������ǣ�____��
��3����NO�ܱ�Na2O2��ȫ���գ�Eװ���е�����Ϊ___________________________��
��4��������ƿA�з�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��5��Cװ�õ�������_____________________________________��
������ʵ���ҳ���Na2SO3������Ũ���ᷴӦ��ȡSO2
��6���������Ƶ�SO2�ı�����Һ�������һ����ʵ�飬�Ƚ�SO2��Fe2+ ��ԭ�Ե�
ǿ�����ɹ�ѡ����Լ��У�SO2�ı�����Һ��FeCl2��Һ����ˮ��FeCl3��Һ��KSCN
��Һ��Ba(NO3)2��Һ��BaCl2��Һ����Ҫ��Ҫд��ʵ�鲽�衢����ͽ��ۡ�_________________________________________________________________��
��7��ijͬѧ�ⶨ���ֱ��ʵ�Na2SO3��Ʒ��Ʒ��Na2SO3�ĺ�������֪������������IO3���ܽ�SO32������ΪSO42����������ԭΪI������
���õ�����ƽ��ȡ16.00g Na2SO3�������l00 mL��Һ��ȡ25.00mL����ƿ�У������뼸�ε�����Һ��
����0.1000 mol L-1����KIO3��Һ�������ữ���ζ�������ƽ��ʵ���ñ�Һ�����Ϊ24.00mL����ζ��յ�ʱ��ƿ�в���������Ϊ________________________��д��������յ������йط�Ӧ�����ӷ���ʽ________________________________����Ʒ��Na2SO3����������Ϊ_________����������������λ��Ч���֣�
���𰸡� ��Һ©�� ˮ����H2O ���Ը��������Һ����ɫ������Һ��ɫ����ȥ C+4HNO3(Ũ)![]() CO2��+4NO2��+2H2O ����NO,��ȥ������̼(���ȥˮ��CO2) ȡԼ2mLSO2������������������Һ���Թ��У��μ�3��5�Σ����������Ȼ�����Һ��ҡ�ȣ��μ�2��3��KSCN��Һ����Һ����죬�ٵμӼ���BaCl2��Һ��������ɫ������˵����ԭ�� SO2��Fe2+�� ��Һ������ɫ���������Ұ�����ڲ���ɫ����ɫ�� 6H++5I-+IO3- = I2+3H2O 22.68%
CO2��+4NO2��+2H2O ����NO,��ȥ������̼(���ȥˮ��CO2) ȡԼ2mLSO2������������������Һ���Թ��У��μ�3��5�Σ����������Ȼ�����Һ��ҡ�ȣ��μ�2��3��KSCN��Һ����Һ����죬�ٵμӼ���BaCl2��Һ��������ɫ������˵����ԭ�� SO2��Fe2+�� ��Һ������ɫ���������Ұ�����ڲ���ɫ����ɫ�� 6H++5I-+IO3- = I2+3H2O 22.68%
����������1������a�Ƿ�Һ©������
��2��̼��Ũ���ᷴӦ�IJ����Ƕ�����������ʵ��Ҫ̽������һ�������ܷ�Na2O2��ȫ��������Ӧ�跨�ö���������ˮ��Ӧת��ΪNO�����Bƿ��װ��������ˮ������H2O����
��3����NO�ܱ�Na2O2��ȫ���գ�Eװ���е�����Ϊ���Ը��������Һ����ɫ��������ɫ����
��4��������ƿA�з�����Ӧ�Ļ�ѧ����ʽΪC+4HNO3(Ũ)![]() CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O��
��5��Cװ�õ������Ǹ���NO����ȥ������̼(���ȥˮ��CO2)��
��6��Ҫ�Ƚ�SO2��Fe2+ ��ԭ�Ե�ǿ�������Ը���������ԭ��Ӧ�Ĺ������һ����Ӧ����SO2����ԭ����Fe2+ ����ԭ����������ṩ���Լ�����������ʵ����ȡԼ2mLSO2������������������Һ�����Թ��У��μ�3��5�Σ����������Ȼ�����Һ��ҡ�ȣ��μ�2��3��KSCN��Һ����Һ����죬�ٵμӼ���BaCl2��Һ��������ɫ������˵����ԭ�� SO2��Fe2+��
��7��ijͬѧ�ⶨ���ֱ��ʵ�Na2SO3��Ʒ��Ʒ��Na2SO3�ĺ�������֪������������IO3���ܽ�SO32������ΪSO42����������ԭΪI������
���õ�����ƽ��ȡ16.00g Na2SO3�������l00 mL��Һ��ȡ25.00mL����ƿ�У������뼸�ε�����Һ��
����0.1000 mol L-1����KIO3��Һ�������ữ���ζ�������ƽ��ʵ���ñ�Һ�����Ϊ24.00mL���ζ��յ�ʱ��IO3����I��������Ӧ����I2����������������������ƿ�в���������Ϊ��Һ������ɫ���������Ұ�����ڲ���ɫ����ɫ����������յ������йط�Ӧ�����ӷ���ʽΪ6H++5I-+IO3- = I2+3H2O���ɵ���ת���غ�õ���ϵʽ6e-~3Na2SO3~ KIO3������n(Na2SO3)=3n(KIO3)=3![]() mol����Ʒ��Na2SO3����������Ϊ
mol����Ʒ��Na2SO3����������Ϊ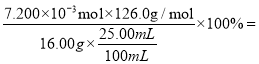 22.68%��
22.68%��