��Ŀ����
3���±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١�⑫�ڱ��е�λ�ã���ش��������⣮| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| һ | �� | |||||||
| �� | �� | �� | �� | �� | ||||
| �� | �� | �� | �� | �� | �� | ⑪ | ⑫ |
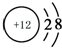 ��
����2���ݡ��ޡ�⑪��ԭ���а뾶�����Ǣޣ�д��ţ���
��3���͢����۵��������ˮ�����м�����Щ����Al��OH��3�����ѧʽ��
��4���ܡ������̬�⻯���е��ȶ���ǿЩ����H2O�����ѧʽ��
��5��д���ں�⑫��ɵĸ�ԭ���������Ӷ�����8���ӵ����ʵĻ�ѧʽCCl4��
��6��д��һ���ɢ٢�����Һ��зǼ��Լ������ʵĵ���ʽ
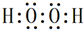 ��
����7���۵���ۺ������ϡ��Һ��ͭ�۷�Ӧ�����ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��8�����ý�ǿ�����Ƴ��������ԭ����д��һ����֤���ں͢�ǽ�����ǿ����һ��������Ӧ�Ļ�ѧ����ʽNa2SiO3+H2O+CO2=H2SiO3��+Na2CO3��
���� ����Ԫ�������ڱ��еķֲ���������֪����H������C������N������O������F������Na������Mg������Al������ Si������P��⑪��S��⑫��Cl��
��1������Mg��þʧȥ���2�������γ����ӣ��������������Ӳ㣻
��2�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С��
��3��ͬ����Ԫ�ص�����������Ӧˮ������������ǿ��ͬ��������������Ӧˮ��������������
��4��Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ���
��5������C��⑫��Cl����ԭ����̼ԭ���γ�һ�Թ��õ��Ӷԣ�̼�����4�����ӣ�����һ��̼���Խṹ4����ԭ�ӣ���������8�����ȶ��ṹ��
��6��������γ�˫��ˮ�к��зǼ��Լ���
��7���۵���ۺ������ϡ��ҺΪϡ���ᣬ��ͭ�۷�Ӧ����������ͭ��һ��������
��8����������̼ͨ���������Һ�У��й����������������˵��̼�������ǿ�ڹ��ᣬ��̼Ԫ�صķǽ���ǿ�ڹ裮
��� �⣺����Ԫ�������ڱ��еķֲ���������֪����H������C������N������O������F������Na������Mg������Al������ Si������P��⑪��S��⑫��Cl��
��1������Mg��þʧȥ���2�������γ����ӣ��������������Ӳ㣬����þ���ӽṹʾ��ͼΪ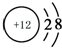 ��
��
�ʴ�Ϊ��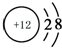 ��
��
��2�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С�����Ԣݡ��ޡ�⑪��ԭ���а뾶�����Ǣޣ�
�ʴ�Ϊ���ޣ�
��3��ͬ����Ԫ�ص�����������Ӧˮ������������ǿ��ͬ��������������Ӧˮ������������������Na������Al�����Ԣ͢����۵��������ˮ�����м�����Щ����Al��OH��3��
�ʴ�Ϊ��Al��OH��3��
��4��Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ������ķǽ�����ǿ���ף�����ˮ���ȶ���ǿ�����⣬
�ʴ�Ϊ��H2O��
��5������C��⑫��Cl����ԭ����̼ԭ���γ�һ�Թ��õ��Ӷԣ�̼�����4�����ӣ�����һ��̼���Խṹ4����ԭ�ӣ���������8�����ȶ��ṹ�����Ի�����Ļ�ѧʽΪCCl4 ��
�ʴ�Ϊ��CCl4��
��6��������γ�˫��ˮ�к��зǼ��Լ��������ʽΪ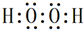 ��
��
�ʴ�Ϊ��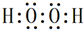 ��
��
��7���۵���ۺ������ϡ��ҺΪϡ���ᣬ��ͭ�۷�Ӧ����������ͭ��һ����������Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��8����������̼ͨ���������Һ�У��й����������������˵��̼�������ǿ�ڹ��ᣬ��̼Ԫ�صķǽ���ǿ�ڹ裬��Ӧ�Ļ�ѧ����ʽΪNa2SiO3+H2O+CO2=H2SiO3��+Na2CO3��
�ʴ�Ϊ��Na2SiO3+H2O+CO2=H2SiO3��+Na2CO3��
���� ���⿼��Ԫ�ص��ƶϣ���Ŀ�ѶȲ�����Ԫ�������ڱ��е����ʿ��ƶϳ�Ԫ�ص����࣬���в����������ɵ�Ӧ�ã�ѧϰ��ע��������֪ʶ��

| A�� | 2s22p2 ��2s22p4 | B�� | 3s23p4 ��2s22p4 | C�� | 3s2��2s22p5 | D�� | 3s1��3s23p5 |
| A�� | 111 | B�� | 272 | C�� | 2007 | D�� | 161 |
| A�� |  | B�� |  | C�� |  | D�� |  |
�ٽ�32.2g Na2SO4��10H2O��������ˮ�У��ټ�ˮϡ����100mL
�ڽ�14.2g Na2SO4����100mLˮ��
�۽�20mL 0.5mol/L Na2SO4��Һ��ˮϡ����100mL��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �٢ڢ� |
| A�� | C2H4 | B�� | NaOH | C�� | MgCl2 | D�� | HCl |
| A�� | NaOH���Ա�KOHǿ | |
| B�� | Li������õĽ�����F������õķǽ��� | |
| C�� | X2+���������ĿΪ18����X�ڵ������ڵڢ�A�� | |
| D�� | Ԫ�����ڱ���7�����壬7�����壬1��0�壬1�����壬��16������ |