��Ŀ����
14����1����ȩ������ˮ��ԭ���Ǽ�ȩ��ˮ���Ӽ��γ��������2���е㣺�״�����ȩ���������������ԭ�״����Ӽ��������
��3��Ϊ�˼�������ЧӦ����ѧ����Ʒ�Ӧ��CO2+4H2��CH4+2H2O�Լ�С������CO2������2mol CH4���ɣ�����12mol�Ҽ���4mol�м����ѣ�
��4���������־��壺��CO2����NaCl����Na����Si����CS2�����ʯ�����ǵ��۵�ӵ͵��ߵ�˳��Ϊ�٢ݢۢڢܢޣ�����ţ���
���� ��1�����Ӽ��γ���������ʵ��ܽ������
��2���״���״����Ӽ������������Էе��쳣�ĸߣ�
��3�������ж�CO2��H2�����к��ж��٦ļ��ͦм������ݻ�ѧ����ʽ���㣻
��4�����ݾ������ͷ������۵㣺ԭ�Ӿ��壾���Ӿ��壾�������壾���Ӿ��壮
��� �⣺��1����ȩ��ˮ���Ӽ��γ���������Լ�ȩ������ˮ���ʴ�Ϊ����ȩ��ˮ���Ӽ��γ������
��2���״���״����Ӽ������������Էе㣺�״����ڼ�ȩ���ʴ�Ϊ�������״����Ӽ��������
��3��1��CO2��4��H2�����й�����6���ļ���2�м�������2molCH4���ɣ�����12mol�ļ���4mol�м����ѣ��ʴ�Ϊ��12��4��
��4�����ݾ������ͷ�����ԭ�Ӿ��壾���Ӿ��壾�������壾���Ӿ��壬Si�ͽ��ʯ����ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ���۵�Խ�ߣ�CO2��CS2���Ƿ��Ӿ��壬��Է�������Խ���۵�Խ�ߣ�Na���۵����100�棬�����۵�͵��ߵ�˳��Ϊ���٢ݢۢڢܢޣ��ʴ�Ϊ���٢ݢۢڢܢޣ�
���� ���⿼��������Ĵ��ڶ��������ʵ�Ӱ��;�����й����ʣ���Ҫ�����˾������͵��жϼ�������������ʣ����ؿ���ѧ���������⡢����������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
4�������£�pHΪ3��FeCl3��Һ��pHΪ11��Na2CO3��Һ��pHΪ3����������ˮ���������H+��Ũ�ȷֱ�Ϊ��C1��C2��C3������֮��Ĺ�ϵ�ǣ�������
| A�� | C1��C2��C3 | B�� | C1=C2��C3 | C�� | C1��C2��C3 | D�� | ���ж� |
2����֪������Ԫ�أ�1��18��Ԫ�أ�������aA2+��bB+��cC3-��dD-������ͬ�ĵ��Ӳ�ṹ��������������ȷ���ǣ�������
| A�� | ԭ�Ӱ뾶��A��B��C��D | B�� | ԭ��������d��c��b��a | ||
| C�� | ���ʵĻ�ԭ�ԣ�A��B��D��C | D�� | ���Ӱ뾶��C��D��B��A |
19��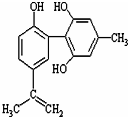 ���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������
���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������
 ���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������
���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������| A�� | �����ʵķ���ʽΪC16H15O3 | |
| B�� | �������뱽������ͬϵ���FeCl3��Һ����ɫ | |
| C�� | �÷����е�����̼ԭ�Ӳ����ܹ�ƽ�� | |
| D�� | 1mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ�������Br2��H2Ϊ4mol ��7mol |
6�������л����У�������������������ǣ�������
| A�� | �ȶ��� | B�� | �ױ� | C�� | ������ | D�� | ���� |
3���±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١�⑫�ڱ��е�λ�ã���ش��������⣮
��1�������ߵ����ӽṹʾ��ͼ ��
��
��2���ݡ��ޡ�⑪��ԭ���а뾶�����Ǣޣ�д��ţ���
��3���͢����۵��������ˮ�����м�����Щ����Al��OH��3�����ѧʽ��
��4���ܡ������̬�⻯���е��ȶ���ǿЩ����H2O�����ѧʽ��
��5��д���ں�⑫��ɵĸ�ԭ���������Ӷ�����8���ӵ����ʵĻ�ѧʽCCl4��
��6��д��һ���ɢ٢�����Һ��зǼ��Լ������ʵĵ���ʽ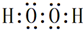 ��
��
��7���۵���ۺ������ϡ��Һ��ͭ�۷�Ӧ�����ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��8�����ý�ǿ�����Ƴ��������ԭ����д��һ����֤���ں͢�ǽ�����ǿ����һ��������Ӧ�Ļ�ѧ����ʽNa2SiO3+H2O+CO2=H2SiO3��+Na2CO3��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| һ | �� | |||||||
| �� | �� | �� | �� | �� | ||||
| �� | �� | �� | �� | �� | �� | ⑪ | ⑫ |
 ��
����2���ݡ��ޡ�⑪��ԭ���а뾶�����Ǣޣ�д��ţ���
��3���͢����۵��������ˮ�����м�����Щ����Al��OH��3�����ѧʽ��
��4���ܡ������̬�⻯���е��ȶ���ǿЩ����H2O�����ѧʽ��
��5��д���ں�⑫��ɵĸ�ԭ���������Ӷ�����8���ӵ����ʵĻ�ѧʽCCl4��
��6��д��һ���ɢ٢�����Һ��зǼ��Լ������ʵĵ���ʽ
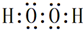 ��
����7���۵���ۺ������ϡ��Һ��ͭ�۷�Ӧ�����ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��8�����ý�ǿ�����Ƴ��������ԭ����д��һ����֤���ں͢�ǽ�����ǿ����һ��������Ӧ�Ļ�ѧ����ʽNa2SiO3+H2O+CO2=H2SiO3��+Na2CO3��
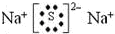 ��
��